Multiple Drug Resistance
Biologist Rajendra Prasad on drug-resistant pathogens, how they affect us and why it is so difficult to fight them
faq | August 24, 2019
Multidrug Resistance or MDR is defined as when an organism displays increased resistance to multiple antibiotics and thus becomes difficult to treat. Multidrug resistance has become a threat to public health due to the extensive spread of drug-resistant pathogens.
When was MDR discovered?
In the 1940-s, when penicillin was first discovered, it was supposed to become a miracle drug to cure all kinds of infections. It was soon after that scientists discovered a disturbing phenomenon that was called Multidrug Resistance (MDR for short).
MDR has been with us (and microorganisms) throughout the existence of antibiotics and antifungals, although the genes responsible for MDR have been there for a much longer time. For instance, MDR genes are found in bacteria recovered from over 30-40,000 years old permafrost deposits, and thus antibiotic resistance can be called ancient. These genes allow bacteria to adapt to contemporary drugs. At first, it took bacteria several years to become resistant to penicillin, but in subsequent discoveries of new drugs, bacteria started developing at a much faster pace.
Although MDR is an evolutionary phenomenon, humans have contributed to its increase through the unsupervised usage of antibiotics in agriculture, medicine, and personal use. In the modern world, drug-resistant pathogens and even superbugs (organisms resistant to all drugs) are being easily spread around the world.
What organisms can become resistant to drugs?
MDR is not restricted to bacterial infections; it is also quite common in fungal diseases. Interestingly, cancer is also drug-resistant. Resistance strategies adopted by bacteria, fungi, and cancer cells are very similar. What’s more, cancer cells can also be compared to a superbug, as almost 50% of cancer cells don’t respond to any anti-cancer drugs. Any drug which is toxic to cancer cells is also toxic to ordinary cells as well, hence further complicating cancer treatment.

As evolution proceeds, many new fungal species are emerging. In 2009, scientists discovered a new type of yeast that has become a global health threat. The yeast Candida auris is different from other fungi as it is intrinsically resistant to drugs (antifungals), which means that while other species develop resistance upon drug exposure, Candida auris has been found to be resistant to drugs from the very beginning.
Fortunately, the percentage of fungi resistant to drugs is 6-10%, and out of 2 million fungal species, only a few hundred are pathogens to animals, plants, and humans. Nonetheless, it is very difficult to treat fungal infections because, as compared to the availability of a large number of antibiotics, antifungal drugs are very few, and new drugs need to be discovered.
How is MDR acquired by fungi?
A fortunate scenario in pathogen-drug interaction is the following: a sensitive cell is being exposed to an antifungal drug, and the drug reaches the target and kills the cell. But in reality, things can go wrong: many drugs don’t kill fungi but only inhibit their growth. These kinds of drugs are called fungistatic (as opposed to fungicides that actually destroy yeast cells), and these drugs open up an opportunity for fungal cells to develop resistance to drugs. Once developing resistance, fungal cells never return to their sensitive state, even in the absence of drugs.
For a living fungal cell, there are various mechanisms to develop tolerance to drugs. By means of genetic mutation or gene expression, fungi can modify or overproduce the target or mutate it. That prevents the drug from binding with the cell target. Another strategy is to increase the production of efflux proteins, which are initially few in a sensitive cell.
There is a superfamily of proteins that belongs to two classes. These proteins are commonly found in bacteria, fungi, plants, animals, and humans. The first one is called ABC. The name comes from ATP-binding cassettes binding proteins (ABC). In humans, the blood-brain barrier is guarded by ABC proteins which ensure that nothing unnecessary gets into the brain. The other one is called the major facilitator superfamily (MFS). Some of the proteins from both superfamilies function as drug exporters and have roles in MDR. While ABC proteins use direct cellular energy to throw the drugs out, MFS proteins act as an antiporter. Nonetheless, both export drugs but apply a different strategy to efflux.
When the amount of efflux proteins is high, as in resistant cells, the drug continuously rapidly effluxes out and, as a result, cannot be retained inside of the cell. The absence or lower concentration of drugs in cells enables them to survive at a higher dose of drugs.
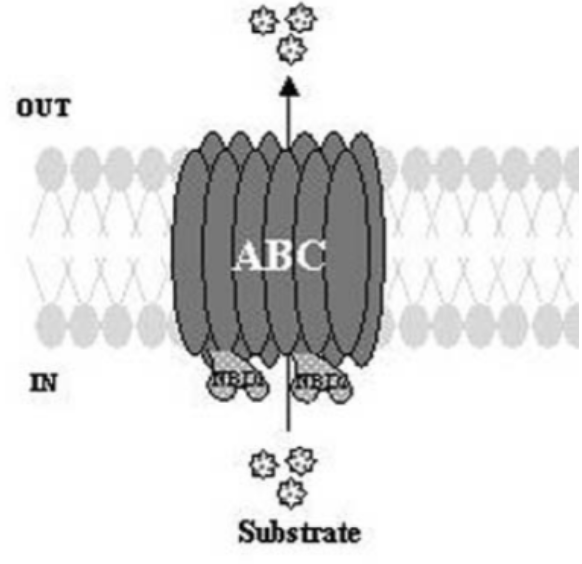
Representation of Efflux transporters of the ATP-binding cassette superfamily (ABC). Prasad, R. et al. “Efflux pumps in drug resistance of Candida.” Infectious Disorders-Drug Targets (Formerly Current Drug Targets-Infectious Disorders) 6.2 (2006): 69-83.
How to deal with resistant pathogens?

Another approach to fighting fungal pathogens is iron depletion. Iron is extremely important for any organism, and iron deficiency leads to serious consequences. It has been discovered that iron chelation makes yeast cells susceptible to drugs.
Why is it difficult to fight MDR?
The process of antifungal development is complicated by the fact that fungi share mammalian metazoan biology. This means that despite our organ system being highly coordinated, it has similar biology to fungal cells. When developing new drugs, scientists have to look for a target in fungal cells that would be unique to the species so that the drug doesn’t affect human cells.
So what is it that fungal cells have and we don’t? Specific to fungi are thick cell walls that aren’t present in bacterial and animal cells. Being a unique component of fungal cell walls makes chitin a perfect target that can’t be found in human cells. A good antifungal prevents fungal cell wall synthesis, which eventually causes fungal cells to die and doesn’t harm the host.
Do fungi have natural enemies?
It is reasonable to assume that a means of fighting fungal diseases must have already been created by nature, and there must be some sort of natural antifungals, as is the case with antibiotics. Luckily for fungi, and quite unfortunately for humans and other species, there is no such thing as a natural antifungal. But it doesn’t mean that there is nothing that can save us from fungal diseases: the most powerful weapon against them is our immune system. Every day, trillions of microorganisms invade our bodies and yet don’t kill us. In fact, it’s them who are being killed by our immune system cells called macrophages. A healthy individual is resistant enough to fungal and bacterial pathogens.
Who is at the most risk for drug-resistant pathogens?
There are manifold fungal and bacterial species that reside in the human body, causing no harm to the host. But if the host is immune-compromised (that is, has undergone surgery or is an HIV patient), these microorganisms can become very dangerous. Fungi can get into the blood system and cause systemic infections. In such cases, the mortality rate can go up to 40%.
Who else suffers from MDR?
There is a small population of amphibians that are infected by drug-resistant fungal pathogens and are now disappearing as there are no natural antifungals. Similarly, marine and coral life are on the verge of extinction because of fungal infections. In total, in the last 30-40 years, the number of plant and animal species targeted by fungal pathogens has been increasing due to the contemporary environmental situation, the way we manage our ecosystems and massive use of antibiotics.
Edited by Karina Akopyan