Oxygen sensor molecules assist breast cancer cells to disseminate
Blocking an oxygen sensor in a breast cancer tumor impairs its ability to metastacize
Serious Science - news | September 7, 2015
In August Cell Reports published an article called “The Cancer Cell Oxygen Sensor PHD2 Promotes Metastasisvia Activation of Cancer-Associated Fibroblasts”. We asked the authors, Prof Mieke Dewerchin, Prof Peter Carmeliet and Dr Anna Kuchnio from the Laboratory of Angiogenesis and Neurovascular Link, Vesalius Research Center, to comment on their research.
The study

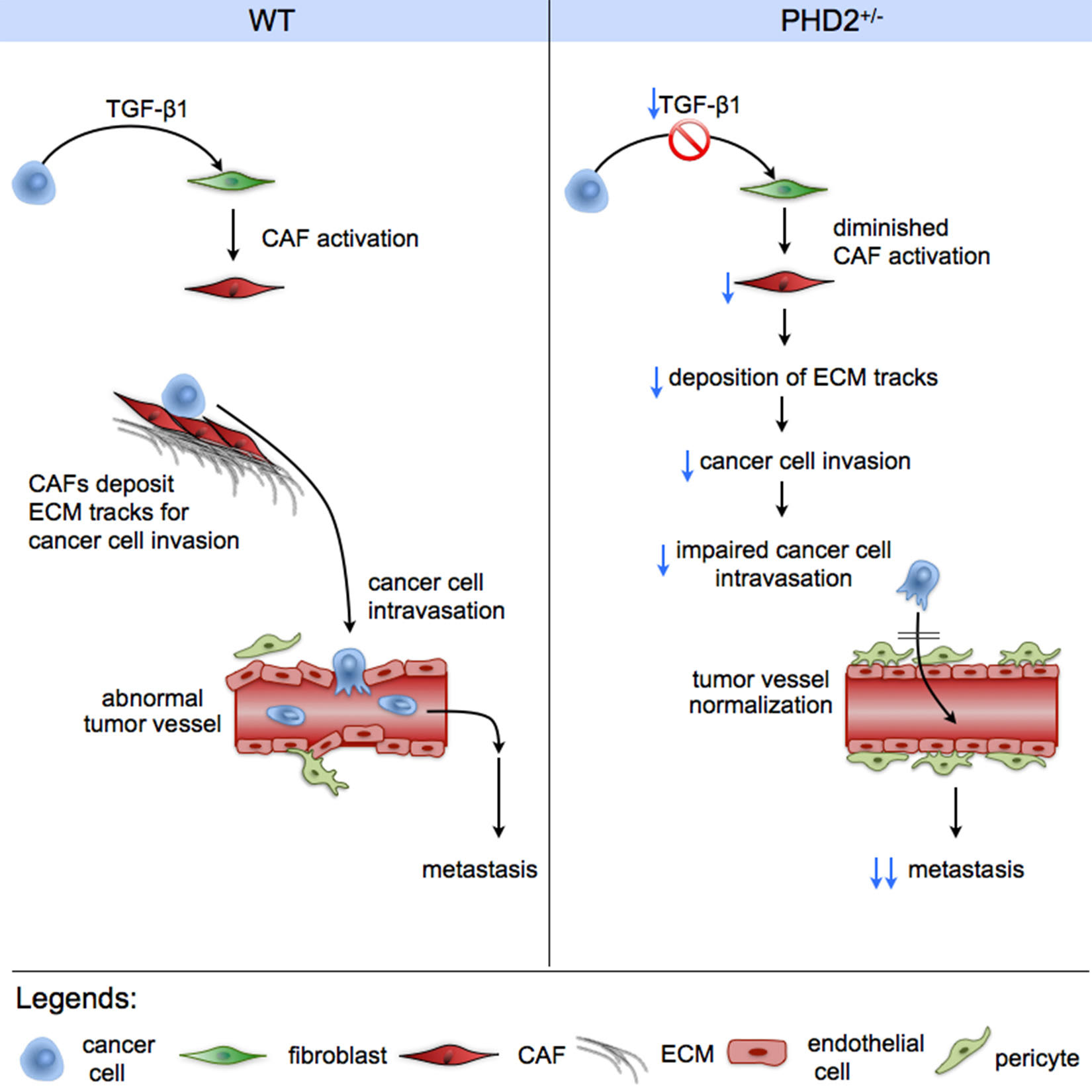
Figure 1: Loss of one PHD2 allele (PHD2+/-) reduces the activation of cancer-associated fibroblasts (CAF) and extracellular matrix (ECM) changes in tumors, and strengthens (“normalizes”) the tumor blood vessels. This dual effect results in reduced cancer cell escape and metastasis. Reprinted from Cell Reports 2(6), Kuchnio et al, “The cancer cell oxygen sensor PHD2 promotes metastasis”, pp 992-2005, Copyright 2015, with permission from Elsevier.
Background
This study was part of our priority research line into the molecular mechanisms underlying the formation of blood vessels in health and disease. We previously had shown a role of PHD2 in normalization of malformed tumor blood vessels and in tumor metastasis by using mouse models of transplanted cancer cells. Other groups also studied the role of PHD2 in cancer cells using tumor xenograft models, reporting opposing effects on tumor growth, likely due to differences among the tumor models and/or the approaches used to block PHD2 (general inhibition, cell type selective inhibition, etc). Of note, none of these previous studies used models of spontaneous tumor formation as occurs in patients. In this study, we wanted to address the role of PHD2 in a mouse model of spontaneous breast cancer, the most frequent cancer and second leading cause of cancer death in women, primarily due to metastasis.
Future directions
The discovery of the prominent dual role of the oxygen sensor PHD2 in CAF activation and tumor vessel normalization facilitating breast cancer metastasis provided important novel insights with potential clinical implications. To explore the clinical relevance, we also blocked PHD2 at a later time point, after tumor onset, to mimic better the patient situation. Also in these conditions, metastasis was reduced. Furthermore, analysis of patient databases revealed that lower levels of PHD2 in human breast cancer samples correlated with reduced expression of markers of CAF activation. Together, our findings warrant further investigation of the potential of inhibiting the CAF-dependent pro-metastatic activity of PHD2 for therapy of tumor metastasis.
If you would like to contribute your own research, please contact us at [email protected]