Prions: Diseases and Treatment
Molecular biologist Byron Caughey on the structure of prions, prion diseases, and their causes and treatment
faq | January 31, 2017
Prions comprise a distinctive class of infectious agents that are protein-based and lack a specific nucleic acid genome. The prion concept includes novel protein-based elements of inheritance, that is, altered forms of host proteins that, when transferred to a new host, can cause a heritable phenotypic change in the recipient. Most prions are pathogens, but compared to all other pathogens (e.g., viruses, bacteria, fungi, parasites), prions are unique in that they propagate within and between hosts without carrying or replicating any DNA or RNA genes of their own.
Prions are typically refolded and aggregated proteins that propagate themselves by incorporating and inducing the refolding of the corresponding normal form of the protein in the host. The prion aggregate grows and then is fragmented somehow to generate more prion aggregates. In many ways, like Kurt Vonnegut’s “Ice Nine”, the growth of prions is analogous to the templated growth of crystals, and prions are often described as “seeds” by analogy to seed crystals. Thus, prions need not carry any nucleic acid code for themselves, but a susceptible host must make the normal precursor protein from which the infectious prions are made. Many, if not most, proteins can refold and/or assemble into ordered aggregates that can seed further growth under certain natural or experimental conditions, whether in a tissue or a test tube. However, not all such protein aggregates are infectious prions. The term prion implies that at the biological level, the refolded state of the protein can propagate between hosts, or at least from cell to cell within a multicellular host. There have been recent calls to include all protein states that promote their own growth as multimeric assemblies under the prion umbrella, but this broadened usage of the term neglects the core concept of transmissibility and fails to discriminate prions from many cellular structures that can grow but have no tendency to spread to other cells and individuals.
History of Research
The prototypic prion disease was a deadly and mysterious transmissible neurodegenerative disease of sheep called scrapie. Early studies revealed that the scrapie agent was unusually resistant to treatments that disinfect other pathogens and could lay dormant in pastures for years. The scrapie agent’s resistance to radiation, in particular, led to proposals in the 1960s by J.S. Griffith and Tikva Alper that it represents a novel class of pathogen that lacks its own nucleic acid genome and might instead be an abnormal self-replicating form of a protein or membrane. Meanwhile, Carlton Gajdusek’s descriptions of the brain pathology of the human disease kuru in Papua New Guinea led William Hadlow to the observation that kuru looks like scrapie in sheep. Accordingly, Hadlow recommended that kuru be tested for transmissibility from humans to other primates. Gajdusek did so successfully and showed that the Fore people were acquiring kuru during ritual cannibalistic feasts. A striking feature of kuru and other prion diseases that have often obscured their causes is the long incubation periods between the initial infection and the appearance of clinical signs, which, in humans, can exceed four decades.
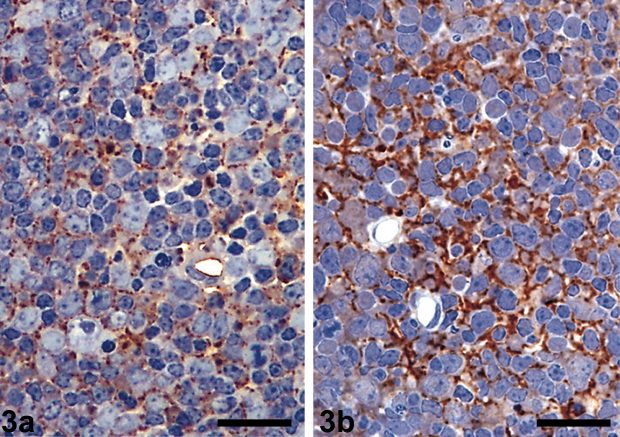
Lymph nodes from (a) healthy and (b) infected sheep – coloring with antibodies shows a clear sign of scrapie prions in the tissue of the infected sheep / wikipedia.org
In the 1980s, Stanley Prusiner coined the term prion for such agents and first identified the specific host protein (prion protein or PrP) that is the main component of scrapie prions. Homologs of the same protein were then found in many mammalian species, including humans, and abnormal PrP aggregates were found in other transmissible scrapie-like neurodegenerative diseases of humans and animals. These diseases are now known as prion diseases or transmissible spongiform encephalopathies. Prusiner, Charles Weissmann, and others showed that PrP is an essential susceptibility factor for prion diseases.
Although the word prion was first applied to the transmissible spongiform encephalopathies described above, the first unequivocal evidence that infectious proteins exist in biology came from Reed Wickner’s realization in 1994 that certain unexplained epigenetic elements of yeast were prions. These prions were not composed of a PrP homolog but of entirely distinct proteins of yeast. The relative simplicity and power of yeast biology and genetics enabled Wickner and others to clearly demonstrate a number of fundamental principles of prion biology and structure that have been much more difficult to pin down with mammalian prion models.
Methods of Research
Unfortunately, many of the standard methods that have long been crucial in studies of conventional pathogens –pathogen-specific genetics, serology, x-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy — have been exceptionally difficult to apply to prions. Without any pathogen-specific genes to sequence and mutate, many of the standard genetic and reverse genetic approaches to revealing pathogen structure and function have not been available. Because prions are composed of host (self) proteins, there is little host immune response to the pathogen and no easy serological, antibody-based way to detect prion infections. Because mammalian prions tend to be tightly packed, heavily glycosylated, and bound to other host molecules, even prion-specific conformational epitopes (surfaces recognized by antibodies) on the PrP aggregates have been difficult to find and exploit. The aggregated yet non-crystalline nature of purified prions has long stymied attempts to determine their 3-D structures.
For many years the only way to detect and measure mammalian prions was by bioassay in animals, which, even in the fastest rodent models, takes months to years for a single experiment. Prion strains were typically discriminated in a given host by comparing incubation periods, neuropathological patterns, and biochemical attributes of the disease-associated PrP deposits or prions.
Fortunately, more recently, powerful cell-free prion amplification assays such as protein misfolding cyclic amplification (PMCA), real-time quaking-induced conversion (RT-QuIC), and the scrapie cell assay have been developed that exploit the inherent replication mechanism of prions. PMCA and RT-QuIC are extraordinarily sensitive and can amplify the presence of prions by a trillion-fold, almost to the point of detecting a few prion particles. PMCA reactions faithfully propagate prion infectivity, reflecting and illuminating many aspects of prion biology. RT-QuIC assays do not, as a rule, propagate fully infectious prions, but provide faster, more practical, higher-throughput methods for detecting prions. As such, they have become state-of-the-art in diagnosing prion diseases. Both PMCA and RT-QuIC can, in some cases, discriminate important prion strains within given host species.
Slow progress is being made in revealing the underlying structure of prions. Solid state NMR studies have revealed molecular architectures of some fungal prions and prion-like fibrillar structures of mammalian PrP. Electron crystallography, fiber diffraction, and cryo-electron microscopic studies have also provided key structural constraints for mammalian prions, but further improvements in the application of these and potentially other structural biological methods are sorely needed.
Structure and Reproduction of Prions
It has been extremely challenging to unravel the structures and replication mechanisms of mammalian prions at least in molecular detail. One must first explain how misfolded host proteins can propagate as pathogens without carrying any of their own nucleic acid-based genetic code. Then one must also explain how proteins of a single amino acid sequence, such as that of PrP in a given host animal, can form different strains of prions that propagate faithfully and cause distinct disease phenotypes –without requiring the sorts of genetic mutations that explain strain variation in conventional pathogens.

Beyond that gross description, the details of prion structure and propagation at the molecular level remain obscure. Also unresolved is how prions propagate beyond the original site of infection in the host. Current evidence suggests that the most efficient transfer between cells involves membranous structures such as exosomes or tunneling nanotubes, most likely because prions are usually bound to membranes by lipid anchors; however, the relevance of these membranous structures to prion spreading in vivo remains to be determined. Spreading mechanisms are important to understand because the relative abilities of various misfolded self-propagating protein aggregates to spread within and between cells, tissues, and individuals are primary determinants of whether they act as infectious pathogens or relatively innocuous accidents of protein metabolism.
Prion Diseases
Many, but not all, mammalian species are susceptible to PrP-based prion diseases, including humans, non-human primates, cattle, sheep, goats, deer, elk, moose, cats, mink, rodents, and various exotic ungulates. Dogs and horses appear to be notable exceptions. Different species usually express slightly different normal PrP molecules, and their differences in PrP amino acid sequence can strongly influence host susceptibility to incoming prion infections. For example, humans are known to be somewhat susceptible to bovine spongiform encephalopathy (BSE) but appear to be resistant to sheep scrapie and, as far as we know, chronic wasting disease in cervids. For some reason, bank voles and squirrel monkeys are unusually susceptible to a wide range of prion infections from other species.
The mechanisms by which prion infections cause neurodegenerative disease are unclear. Different prion strains within a given host type can accumulate preferentially in different regions of the central nervous system and cause a range of neuropathological lesions. Obviously, the ultimate effect of at least some of the damage is the malfunctioning and loss of neurons, causing a variety of clinical signs and death. A number of neurophysiological processes and pathways are known to be disrupted, but much remains to be determined about (i) whether such disruptions are due to direct or indirect toxicities of prions and (ii) the extent to which any given deficit or combination of deficits is most responsible for the ultimate demise of the host.
In humans, the causes of prion diseases can be genetic (due to specific mutations in the host’s PrP gene), acquired (due to infections, such as exposures to kuru, BSE or other prion-contaminated materials), or sporadic (of unknown origin, but usually presumed to be due to spontaneous prion formation in the individual). The vast majority of human prion diseases are sporadic, the most common being sporadic Creutzfeldt-Jakob disease (sCJD), which has an incidence of about 1 case per million population per year worldwide. A number of different mutations in the PrP gene can cause a variety of familial human prion diseases, with some mutations being fully penetrant (always causing disease in people carrying the mutation) and others being less penetrant. The clinical symptoms and progression can vary markedly between prion disease types and individuals but can include dementia, incoordination, insomnia, hallucinations, muscle stiffness, confusion, fatigue, and speaking difficulties.
There are also important prion diseases of animals. BSE arose as a major epidemic in cattle due to what might be described as “agricultural cannibalism” in the 1990s. Consumption of BSE-contaminated beef then caused nearly 200 cases of variant CJD in humans. However, preventative measures have nearly eliminated BSE and the occurrence of new vCJD cases. Chronic wasting disease of cervids is sweeping through North America at an alarming rate, with cases also arising in South Korea and Norway. Scrapie is a persistent problem in sheep and goats in many parts of the world.
Diagnosis and Treatment of Prion Diseases
Considerable strides have been made recently toward being able to diagnose human prion disease accurately and relatively non-invasively in living patients based on new prion-specific testing of cerebrospinal fluid, nasal swabbings, blood, urine, or skin. For example, RT-QuIC testing of cerebrospinal fluid and/or nasal brushings can be nearly 100% accurate in diagnosing sCJD. These tests have the advantage of measuring the causative agent of prion disease but have not yet been fully vetted and recommended officially by organizations such as the WHO. Otherwise, diagnoses of sporadic prion disease in humans depend primarily on a confluence of clinical signs, brain scans, electroencephalograms, and other biomarker measurements, which collectively can have high diagnostic sensitivity, but are not fully specific for prion disease.
Notwithstanding the recent advances in the new prion tests described above, current guidelines indicate that a definitive diagnosis of sporadic or acquired prion disease requires a neuropathological examination of brain tissue obtained by biopsy (rare) or autopsy. I expect that the guidelines will soon be changed to include the new less-invasive intra vitam tests for prions. Unfortunately, although the above improvements in the early definitive diagnosis of prion disease should improve prospects for developing and implementing therapeutics, no treatments are currently available that have proven to be effective in the clinic.
Open Question and Future Directions
Key questions that remain in the mammalian prion disease field are: 1) What are the self-propagating structures of prions, and how do they vary with prion strain? 2) How do prions damage the brain? 3) How can we prevent or repair the damage to treat these diseases? 4) What are the most relevant transmission mechanisms for prion diseases in humans and animals? 5) Which, if any, prion diseases of animals (besides BSE) have zoonotic potential to cause disease in humans? 6) To what extent can other pathogenic misfolded proteins with self-seeding activity behave like PrP-based prions in being able to propagate within or between individuals to cause disease?
These findings raise urgent questions about whether these many protein-misfolding diseases, which are often much more common than the PrP-based prion diseases, might be transmissible in humans or animals under practical, real-life circumstances. CJD has been transmitted between people via tissue transplants, cadaver-derived hormone injections, blood transfusions, and contaminated medical instruments. A contributing factor in such iatrogenic transmissions is the fact that prions are often not fully inactivated by standard clinical disinfection procedures. Whether other types of potentially prion-like disease-associated protein aggregates might be similarly resistant to inactivation and then capable of initiating or accelerating pathogenic processes in people remains to be determined. I know of no epidemiological indications that this is the case, but further scrutiny seems warranted.
Edited by Amir Baikatov