3D Bioprinting Applied for Tissue Engineering
Bioengineer Ali Khademhosseini on the challenge of biocompatibility, biological inks, and printing organs on t...

If you stop someone on the street and ask them what they think about chemistry, chances are, they will come up with a negative image. Most likely, they will think of dangerous experiments, toxic substances and explosions: something about an evil genius in a white lab coat concocting a deadly poison that will wipe out all of humanity. Chemistry is something weird, something unnatural, they might say; something to be avoided at all costs. Why can’t we just stick to what mother nature has already given us?
While this image is powerful, it’s not quite accurate. With all the challenges facing our modern society, from environmental crisis all the way through to overstretched healthcare resources and hunger and undernourishment in the world, chemistry is the science that has a potential to give us the answers to almost any problem we might throw at it. Coming up with a novel protective coating for solar panels? Sure. Figuring out a safer anticancer drug? Absolutely. Developing alternative food sources to ensure more people have access to nutritious food? Happy to help.
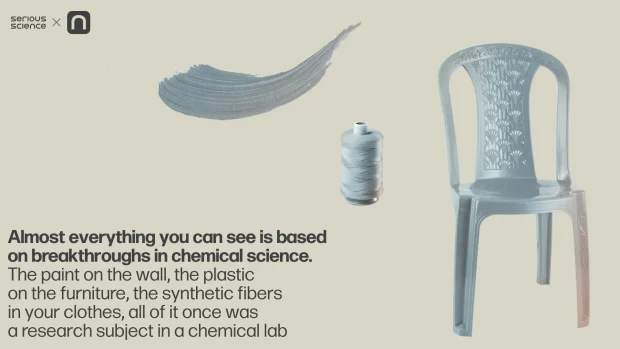
In fact, if you have a problem where the solution would look like a compound with certain predesigned properties, then chemistry is your best friend, as this is what chemistry, essentially, is: exploring the properties of simple and complex substances and figuring out how to create new ones with specific targets and goals in mind. Look around you: almost everything you can see is based on breakthroughs in chemical science. The paint on the wall, the plastic on the furniture, the synthetic fibers in your clothes, all of it once was a research subject in a chemical lab. Chemistry really is one of the key disciplines to enhance, and innovate, and improve society.

While coming up with new materials and drugs might be obvious tasks for chemists to deal with, there’s a lesser-known field of chemical studies that’s nevertheless crucial to every aspect of our lives, namely catalysis. Say, we know that A and B together make a product that we need for polymer production; we mix them but then nothing happens. Why? Because the activation energy, i.e., the amount of energy that’s needed for reaction to happen, is too high. We might try and heat up the chemicals to reach that activation energy, but it may be costly, and it can lead to all sorts of problems — including the notorious lab explosions, so prevalent in our ideas of chemistry.
So instead we can use a catalyst. Catalysts lower the activation energy thus speeding up the reaction, allowing us to produce the desired chemical compound cheaper and faster. It is estimated that about 90% of all chemical products rely on catalysis at some stage of their production [1]. A classic example of the benefits of catalysis is the procedure for industrial ammonia production: in the Haber–Bosch process nitrogen and hydrogen combine to form ammonia, but in order for the reaction to take place you need a catalyst, usually an iron-based one, and even then, high pressures and high temperatures are needed to drive the reaction forward.
Another example of a highly important area where catalysis plays a major role is oil refinement. We start the production with crude oil which consists of various hydrocarbons that need to be separated by fractional distillation to become suitable for further applications. The refinement process relies heavily on catalysis: it is the only way to make it possible and, by extension, to make possible the production of plastics, different types of fuel, various agricultural fertilizers and pesticides, household products such as soaps and detergents, synthetic fibers for our clothes and lots of other products so essential to our daily lives [2]. Without the ability to speed up the reactions we would not have had the world that we live in today.
There’s still a lot of room for improvement in this area: even with existing catalysts there’s always high demand for better alternatives. Some of the requirements that the industry has for new catalysts include their activity, so that less energy would be needed to start the reaction; selectivity, so that the catalyst would only drive the production of the desired compound with no by-products; stability, so that the catalyst would not dissolve mid-way through the reaction; easier separation from the reaction products, so that we wouldn’t need to lose time and money purifying them; and reasonable price. Even if we improve one aspect by 1%, given the scale of industrial production, that’s already a massive amount of money saved and waste reduced. That’s not an easy thing to do, but with the rise of artificial intelligence we can significantly speed up the search for new compounds to allow for a greener economy and sustainable future.

One of the most interesting compounds that can be used in catalysis is palladium. Palladium is the key catalyst for carbon–carbon coupling and carbon–heteroatom coupling, which are essential reactions for producing complex drug molecules in pharmaceutical industries. It has quite some advantages because it mostly works under mild conditions, which means we don’t need to use so much energy to induce the reaction and we can get the end product faster. It also has different oxidation states which is crucial for any catalyst, as it allows us to vary its properties and design the exact compounds we need to enhance the desired reaction. Moreover, it’s very selective, which makes it perfect for working towards greener, environmentally friendly production.
The downside is that palladium is quite costly. One way to overcome this problem would be to develop palladium alloys with cheaper metals: for instance, we could combine palladium with nickel, copper, silver, etc. and thus reduce the cost of the catalyst but keep the excellent performance under mild conditions. Another solution would be to work with heterogeneous catalysts which are easier to separate from the reaction mixture: that allows us to recycle and reuse the catalyst thus making the price of the palladium less of an issue, as we could always reuse it over and over again.
Given the ample range of tasks that chemistry is able to handle, its possible applications in various aspects of our lives are indeed endless. Of course, we still need to work on optimizing production and reducing costs for already existing reactions and technologies, but there are even more interesting tasks for applied chemistry to handle. One of the most crucial areas for future research is energetics: as modern society needs more and more energy, there is increasing demand for alternative energy sources, such as solar power or wind power, and new transportation solutions, such as electric or hydrogen-powered vehicles. Another promising research area lies with renewable biomass energy, with the idea of deriving energy from various types of organic matter, ranging from plants to solid waste.
Naturally, medicinal chemistry is working day and night on developing more efficient, less toxic drugs, including anti-cancer drugs, antiviral drugs, antibiotics and many others; lots of efforts also go into finding cures for diseases that so far have been resisting treatment, such as Alzheimer’s or other types of neurodegenerative disorders. Food safety and proper nutrition can also be ensured with new discoveries in chemistry. New fertilizers and pesticides can help us reduce constraints to agricultural production, while new solutions for post-harvest treatment and storage can reduce waste and thus lessen the ongoing hunger crisis. Fortification of various food vehicles, with the aim to treat or prevent micronutrient deficiencies, can help us build a more balanced diet for those who previously didn’t have access to nutritious foods, while making the solution sustainable and cost-effective [3].

All these tasks could easily be solved with enough time and investment, and yet, the general public is still quite wary of chemistry. Unfortunately, the persistent prejudice might cause problems for our society: a world without chemists is one of the plausible scenarios that the Royal Society of Chemistry is considering in its report on what might be awaiting chemical sciences in the future [4]. The oh-so-very-dangerous chemistry, the poisons-and-toxins chemistry repels young students who end up choosing other fields of studies, thus reducing the number of specialists with special training in chemistry and, consequently, slowing down the research and the development of our society. But wouldn’t it be a shame to miss a simple yet elegant solution to the global food crisis or decarbonization problem simply because we are afraid of science?
1. Fechete I., Wang Ye, Védrine J.C. (2012). The past, present and future of heterogeneous catalysis. Catalysis Today, 189(1): 2-27.
2. Speight J.G. (2019). Handbook of Petrochemical Processes. CRC Press.
3. García-Martínez J., Serrano-Torregrosa E. (2011). The Chemical Element: Chemistry’s Contribution to Our Global Future. Wiley-VCH.
4. Palermo A. Future of the Chemical Sciences. Royal Society of Chemistry.

Bioengineer Ali Khademhosseini on the challenge of biocompatibility, biological inks, and printing organs on t...

Biophysicist Georg Büldt on using fluorescent protein markers, hormonal communication during pregnancy, and in...

Geneticist Vadim Gladyshev on selenoprotein genes, the 21st amino acid, and selenium in human diet