Unique Sound Systems Allow Parrots to Talk
Scientists have found structures in parrot brain responsible for sound imitation

In September the Biophysical Journal published an article called “PE and PS Lipids Synergistically Enhance Membrane Poration by a Peptide with Anticancer Properties” about the effect of the venom of the wasp Polybia Paulista on cancer cell proliferation. We asked one of the authors, Dr Natalia Bueno Leite from São Paulo State University, to comment on this study.
MP1 is a short peptide present in the venom sac of a Brazilian wasp Polybia Paulista. It has been shown that MP1 selectively inhibits cancer (leukemic, prostate and bladder) cell proliferation and so shows promise as an antitumor agent. While the anticancer properties of MP1 were known, the mechanism by which the peptide achieves this was not. The evolution of lipid composition in cancer cells causes changes in the charge and fluidity of their membranes. This could directly affect the interaction of compounds such as bioactive peptides like MP1. The presence of anionic phosphatidylserine (PS) in the outer monolayer of cancer cells could be the origin of its selectivity. However, biophysical studies on model membranes had failed to show a significant enhancement in MP1 disruption of PS-containing membranes, particularly in electrical characterization experiments. Recently, it has been observed that phosphatidylethanolamine (PE) lipids are also externalized to the outer leaflet of tumor endothelial cells due to both lipids being co-regulated by the same transporters. Therefore, we investigated the effects of PS and PE on the capacity of MP1 to disrupt model membranes, to understand the influence of lipid composition on MP1’s affinity for tumor cells. We achieved this by combining circular dichroism, fluorescence spectroscopy, confocal microscopy and atomic force microscopy techniques.
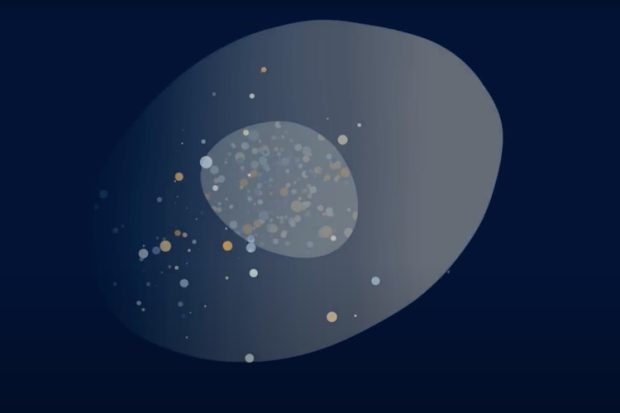
We observed that the presence of PS and PE lipids significantly enhanced membrane disruption by MP1. We found that PS lipids helped the peptide bind to the membrane due to favorable electrostatic attraction. On the other hand, PE lipids were found to increase the membrane’s vulnerability to MP1 resulting in membrane damage due to the formation of large holes that made their structure leaky. The combination of PS and PE lipids were found to be critical due to their complementary effects: the PS causes MP1 to accumulate on the membrane and the PE increases its sensitivity to being “ripped open” by MP1. Confocal microscopy experiments on cell-sized model membranes proved that once the barrier properties provided by these lipids had broken down, large molecules could quickly pass across the membrane. In a real cell, this would result in the leakage of vital chemicals such as RNA and proteins. The breakdown of these membranes was even more compellingly seen using atomic force microscopy on model membranes that were formed on top of a supporting surface. Strikingly, holes were seen to form in membranes containing both PE and PS almost immediately after the addition of the MP1 peptide and these were larger and in greater number than any membrane defects that occurred over much longer timeframes in membranes of other compositions. Taken together, our findings demonstrate that pathological changes in the lipid distribution in cancer cells could form the mechanistic basis for MP1’s antitumor properties. Importantly, no current cancer medications target these changes in cancer cell membranes. Therefore, the MP1 mechanism of action could be a guide in the development of novel cancer therapies with high efficiency and low toxicity to healthy cells.
Bioactive peptides have been studied from the last two decades, especially as good candidates for new antibiotic therapies. This is because they act selectively on bacterial cells, independently of chemical receptors, by damaging the microbial cytoplasmic membrane and causing cell disruption or cell death through leakage of the cell’s inner contents. Among them are peptides with anticancer activity such as MP1. About ten years ago we started to study the effects of MP1 as an antimicrobial peptide on model membranes. But, around 2008 a Chinese group showed its activity in the inhibition of bladder and prostate cancer cells. Then, we started to think about how the peptide might possess a mechanism of selectivity in this scenario. We initially designed experiments on model membranes containing PS lipids in an attempt to simulate the electrostatic nature of a cancer cell. We were able to observe the high affinity of MP1 for these model membranes, but we were also able to conclude that this was not sufficient to explain the antitumour activity of the peptide by itself. So, by including PE lipids in the model membranes and performing advanced biophysical imaging experiments such as confocal and atomic force microscopies, it enabled the direct observation of MP1’s effect under conditions that mimic the membrane properties of cancer cells. This showed the importance of the synergistic effect of PS and PE lipids in the cell’s lipid composition that promote’s MP1’s destructive action on the membrane.
MP1 has not evolved to efficiently target and destroy tumour cells and so is unlikely to be opttimised for this purpose. Therefore future research in this area will include studies that modify MP1’s amino acid sequence in an attempt to make it even more potent and selective to cancer cells. Now that we believe we understand the mechanism by which the peptide works, this will help guide rational choices in designing modified variants of MP1 to test in further laboratory studies.

Scientists have found structures in parrot brain responsible for sound imitation
Professor of Physical Chemistry Peer Fischer on nanorobots, why are they so hard to work on and what might be ...

Bioengineer James Collins on genetic toggle switch in E. coli, bacteriophage, and engineered probiotics