Predicting Functional Effect of Human Mutations
Harvard Associate Prof. Shamil Sunyaev on protein evolution model, human disease mutations, and the help of di...

Modern medicine recognizes three primary methods for treating cancer: surgery, radiation therapy, and drug therapy. While surgery and radiation offer significant benefits, they have a critical limitation: their effects are localized. If cancer has metastasized to other organs by the time treatment begins, removing the primary tumor cannot prevent a recurrence. To effectively combat cancer, both visible and undetected tumor cells must be eradicated. Drug therapy is best suited for this task, as it targets cancer cells throughout the body. While it may not always eliminate the tumor, it can often transform cancer from an acute disease into a manageable chronic condition.
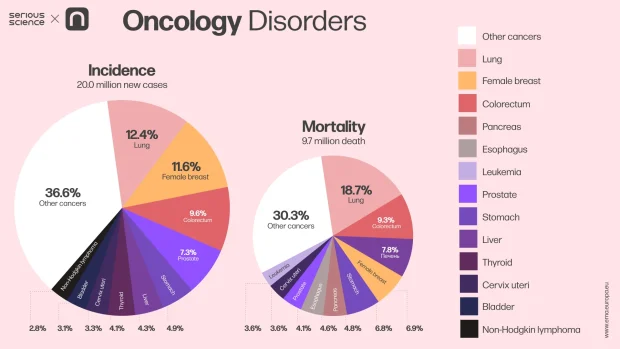
However, drug therapy also comes with its challenges, the most significant being severe side effects. This issue arises because most chemotherapy drugs have low selectivity, attacking all cells indiscriminately. As a result, both cancerous and healthy cells essential for the body’s normal functioning are destroyed. To overcome this problem, drug therapy must employ compounds that specifically target cancer cells without harming the rest of the body. One way to achieve this is by designing drugs that bind to proteins in metabolic pathways unique to tumor cells.
High-specificity molecules, which show great potential for cancer treatment, are typically proteins or low-molecular-weight compounds derived from natural sources, such as secondary metabolites of plants or other organisms. However, these highly specific compounds are often extremely difficult to synthesize in a laboratory, and extracting them from natural sources in quantities sufficient for therapeutic use is often impossible.
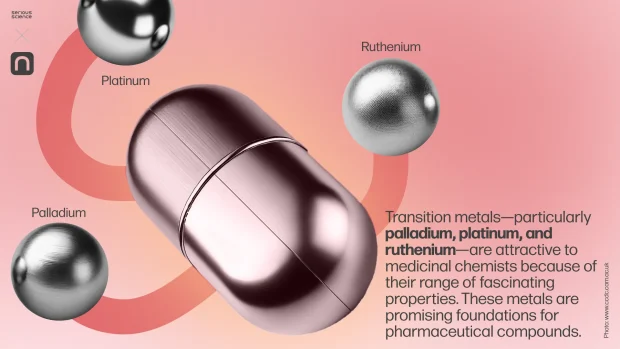
This creates a challenge for medicinal chemistry: synthesising artificial compounds with properties similar to natural anti-cancer substances. Traditionally, such compounds have been organic, but recent developments have explored alternative approaches using compounds based on transition metals.

Transition metals—particularly palladium, platinum, and ruthenium—are attractive to medicinal chemists because of their range of fascinating properties. These metals are promising foundations for pharmaceutical compounds.
Firstly, transition metal complexes can form a greater variety of geometric configurations than carbon-based compounds. This expands the possibilities for drug development: the closer an active substance’s structure aligns with its target biomolecule’s structure and characteristics, the higher its specificity and therapeutic efficacy, and the fewer the side effects. For instance, carbon-based compounds are limited to linear, trigonal, and tetrahedral structures. In contrast, transition metal complexes allow for other configurations, such as octahedral complexes, where six substituents surround a central atom. These six substituent complexes, which have more stereoisomers than carbon-based compounds, can be more precisely tailored to target molecules, ultimately interacting with proteins and nucleic acids in ways that differ from purely organic compounds.
Secondly, the coordination bonds between the metal and ligands in these complexes are generally weaker than the covalent bonds in organic compounds. This makes it easier to create the desired bioactive molecules and, when necessary, modify their composition and structure compared to organic compounds. Moreover, by adjusting the kinetic and electronic properties of the metal center and its ligands, it is possible to control the kinetics of ligand exchange. For instance, the bonds between the metal center and ligands can form and break at varying speeds, from nanoseconds to several decades. This allows for the design of metal-containing drugs with prolonged effects.
Among the wide variety of bioactive complexes, two classes of metal-based agents stand out: functional agents and structural agents [2]. The distinction lies in how they interact with their targets. Functional agents act by covalently bonding the metal to the biological target. These compounds are often prodrugs whose active form develops under biological conditions. Structural agents, on the other hand, interact exclusively through non-covalent interactions.
Despite their potential, metal-based drugs have flaws. Some transition metal complexes exhibit low hydrolytic stability and can behave unpredictably under physiological conditions. However, clinical trials have already confirmed the safety of several metal-containing drugs, gradually alleviating clinicians’ concerns about these pharmaceuticals.
By harnessing the unique properties of transition metals, medicinal chemists continue to unlock new possibilities in drug development, paving the way for more effective and versatile treatments.
Metals dominate the periodic table, accounting for approximately 80% of all known elements. Their vast diversity enables their use across numerous fields, including medicine. Each year, the repertoire of metal-containing molecules expands, encompassing pharmaceuticals and diagnostic agents, tools for bioimaging, and photodynamic therapy applications.
For example, isotopes of rubidium, technetium, indium, and thallium are widely used in diagnostic imaging techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Ruthenium and gallium compounds serve as anti-cancer agents, while samarium, strontium, radium, and yttrium are employed in radiation therapy for malignant tumors [3]. Silver and bismuth compounds exhibit antimicrobial properties, gold compounds are used as antirheumatic agents, and lithium-based medications are renowned for their psychoactive effects. In dentistry, palladium is used to create modern dental prosthetics.
This list could go on indefinitely: Metals are used in virtually every area of medicine. The future of medicinal chemistry is intricately tied to the development and utilization of metal-containing compounds.
Transition metal compounds are already used in clinical oncology. Platinum-based drugs such as cisplatin, carboplatin, and oxaliplatin are widely used to block DNA replication in cancer cells. Additionally, several ruthenium compounds are in the final stages of clinical trials. Recently, the first palladium-based compound, padeliporfin (Tookad), was approved in Europe for clinical use in photodynamic therapy for prostate cancer [4].
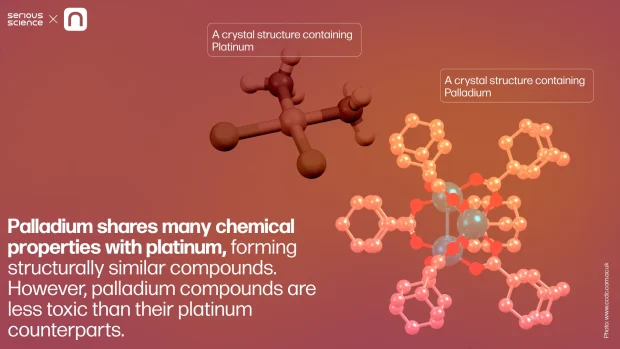
Palladium shares many chemical properties with platinum, forming structurally similar compounds. However, palladium compounds are less toxic than their platinum counterparts. This prompted researchers to explore “palladium analogs of cisplatin,” hoping to achieve the same anti-cancer efficacy with reduced side effects. Unfortunately, these efforts have been largely unsuccessful.
Cisplatin’s anti-cancer activity and high toxicity stem from a unique chemical feature of platinum: its prolonged ligand exchange reactions. This slow reaction binds platinum to DNA’s nitrogenous bases, triggering programmed cell death. In contrast, palladium compounds undergo ligand exchange and degradation far more rapidly in the body, rendering them ineffective for this cancer treatment.
A more promising approach involves using stable palladium compounds that remain chemically intact throughout their action period and interact with biomolecules through non-covalent binding [5]. Active research is currently underway to develop such compounds. Efforts focus on identifying potential drug candidates, investigating their mechanisms of action and metabolic pathways, and evaluating critical factors such as activity, toxicity, and ease of synthesis for large-scale production.
When can we expect results from these studies? Unfortunately, the road to market for such drugs is long. Preclinical and clinical trials alone can take about ten years, not to mention the time required to spark genuine interest from pharmaceutical companies willing to fund the research. While a decade or two is relatively short from a historical perspective, for patients suffering from currently untreatable forms of cancer, this wait can be devastating. However, strategic and timely funding could accelerate the introduction of advanced metal-based therapies into clinical practice.
This article was first published on PostNauka: https://postnauka.org/longreads/157487
1. Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R.L., Soerjomataram, I., & Jemal, A. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a Cancer Journal for Clinicians, 74(3), 229–263.
2. Gianferrara, T., Bratsos, I., & Alessio, E. (2009). A categorization of metal anticancer compounds based on their mode of action. Dalton Transactions, 37, 7588.
3. Mjos, K.D., & Orvig, C. (2014). Metallodrugs in Medicinal Inorganic chemistry. Chemical Reviews, 114(8), 4540–4563.
4. TOOKAD. European Medicines Agency (EMA). 12.07.2022
5. Katkova, S. A., Bunev, A. S., Gasanov, R. E., Khochenkov, D. A., Kulsha, A. V., Ivashkevich, O. A., Serebryanskaya, T. V., & Kinzhalov, M. A. (2024). Metal‐(Acyclic Diaminocarbene) Complexes Demonstrate Nanomolar Antiproliferative Activity against Triple‐Negative Breast Cancer. Chemistry: A European Journal, 30(28).

Harvard Associate Prof. Shamil Sunyaev on protein evolution model, human disease mutations, and the help of di...
Physicist Eric Mazur on semiconductor surfaces, light detection, and the conversion of solar energy into elect...

MIT Professor Robert Langer on artificial organs, fibers encapsulation, and diseases that can't be treated wit...