Cancer Metastasis Promotion by CCR5 Receptor
Biologist Richard Pestell on the cancer GPS, chances to live a long life with the disease, and promising exper...
On January 9, 2015 medical journal Blood published a paper “Programmable 3D silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies” describing new system for studying blood formation and diseases. We have asked one of the authors of this research, Prof. Alessandra Balduini from University of Pavia, to comment on this work.
The study presents the first three-dimensional tissue system that reproduces the complex structure and physiology of human bone marrow and successfully generates functional human platelets. Using a biomaterial matrix of porous silk, the model is capable of producing platelets for clinical use and provides a laboratory tissue system to advance the study of blood platelet diseases.
The special properties of silk protein are essential to successfully mimicking the bone marrow microenvironment. Silk protein possesses a unique molecular structure that enables it to be modeled in a wide variety of forms and stiffnesses, characteristics that have been shown to affect platelet formation and release. Silk is biocompatible and has the ability to stabilize bioactive agents at normal temperatures allowing it to be ‘functionalized’ by adding such agents. Silk is nonactivating to platelets – it does not trigger clotting, allowing the collection of functional platelets from the bioreactor.
The new model combines microtubes spun of silk, collagen and fibronectin surrounded by a porous silk sponge. Megakaryocytes – some of which were derived from patients – were seeded into the engineered microvasculature. We were able to increase platelet production in the bioreactor by embedding the silk with active endothelial cells and endothelial-related molecular proteins that support platelet formation.
Laboratory tests showed that the platelets being generated and recovered from the tissue system were able to aggregate and clot. While the number of platelets produced per megakaryocyte was lower than normally made in the body, the system represents a significant advance over previous models. The scalable nature of the bioreactor system provides engineering options to increase yields of platelets in ongoing studies.
In addition to providing a platform for studying the processes that regulate platelet production, the new system can also provide an in vitro laboratory tissue system to study mechanisms of blood disease and to predict target and efficacy of new drugs.
The system was developed in Italy and the United States, the result of ongoing collaboration among an international research team. In 2008 I visited Tufts to discuss my ongoing studies on blood cells and matrix factors that are important in controlling blood cell functions. During this discussion David Kaplan and me realized there was potentially great synergy between the research in blood cells and diseases and 3D tissue-engineered systems, including ongoing work on silk tubular systems for blood vessels. This led to joint studies, an early model and the current system, which not only yields more platelets, is also more scalable.
Collaborative research building on the strengths of multiple investigators is critical to making this type of progress as well as the involvement of a talented and motivated graduate students and postdoctoral researchers.
The 3D tissue model can provide an in vitro laboratory tissue system to study mechanisms of blood disease and to predict efficacy of new drugs—providing a more precise and less costly alternative to in vivo animal models. Beside platelets this bone marrow model could be used to study the production of other types of blood cells and related diseases. This patient-specific system could provide insight and options for clinical treatments.
In the future, platelets could be generated on demand, avoiding the complications of storage problems. They could also be produced in greater quantities and with better quality and control in terms of morphology and function.
Lastly, the platelets produced can be used as a source of growth factors for wound healing in regenerative medicine, including healing of ulcers and burns, and stimulation of bone tissue regeneration in dentistry and maxillofacial plastic surgery.

Biologist Richard Pestell on the cancer GPS, chances to live a long life with the disease, and promising exper...
Professor of Physical Chemistry Peer Fischer on nanorobots, why are they so hard to work on and what might be ...
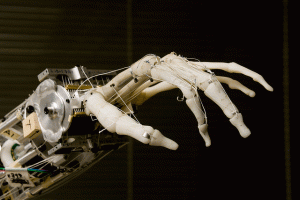
On neurointerfaces, cyber prosthetics' functioning, and external actuators' control