Cognition Without a Cortex
Neuroscientist Onur Güntürkün on mammalian brain, ravens’ ability to plan into future and why birds are so int...
The video is a part of the project British Scientists produced in collaboration between Serious Science and the British Council.
Electron cryomicroscopy makes you solve the tool, the instrument – an electron microscope. Obviously, this could not have been conceived of before the discovery of electrons around about by 1900 by J.J. Thompson. Recently we had the hundredth anniversary of the discovery of the electron. So once it was known that from the wave-particle duality that electrons could be focused just like light waves, the idea of the electron microscope came in with Ernst Rusca. And the great advantage there is that the wavelength of an illuminating field of electrons, rather than light, the wavelength is something like a hundred thousand times smaller. So light has a wavelength of about one micrometer, electrons have a wavelength of one picometer, something like that.
It wasn’t probably until the work of Bob Glaeser who’s currently at Berkeley still, an American scientist. He pointed out that radiation damage – that means the electrons going through the specimen breaking bones and changing the structure from what it was (in biology proteins, and lipids, and so on) into burned products. So you break off hydrogen atoms, they’re given off as a gas, and you’re left with a remnant to what the structure was. Glaeser pointed out that you could never determine the structure of single molecules because of radiation damage. He advocated using crystals, which is a way of finding out how these structures interact with electrons without this…
You’ve got a structure consisting of, perhaps, ten thousand molecules and you can get structural information even though you’ve destroyed a few of them. Glaeser was the very first person with his students at Berkeley to try the idea of overcoming or reducing radiation damage by cooling the specimen. And they cooled it to roughly speaking minus 100 degrees Celsius. That was the farthest they could go, because the electron microscopes had really bad vacuums, it would be contaminated.
It wasn’t until about 1980 that better vacuums, better cold stages – that’s for cooling the specimen to low temperatures – allowed you to reach the temperature of liquid nitrogen. And it was the work of Jacques Dubochet’s group at the European molecular biology laboratory in Heidelberg. He studied the behavior of water, which is the environment in which all of the biological structures are normally found. He studied it for three, four, five years. He studied: when you freeze water, it forms a crystal ice, sometimes the ice is a hexagonal crystal, sometimes it’s cubic, but if you freeze it very rapidly, it forms amorphous ice. And he developed a method for making a thin film by blotting a thin film of it, and plunging it into… initially they tried liquid nitrogen, but because it’s nearly at the boiling point and you have a gas, the cooling is too slow, and you get a crystal.
I think, the true beginning of electron cryomicroscopy came from the work of Dubochet. At that time we were working on 2D crystals of a protein called bacteriorhodopsin. We worked initially at room temperature, we didn’t cool it, by cooling it to liquid nitrogen temperature we could get four or five times as much data from the images before radiation damage. So cooling the specimen doesn’t stop radiation damage, but it stops the consequences of radiation damage by about a factor of four or five. And so we were using electron cryomicroscopy to determine the structure of two-dimensional crystals.
But the real power of electron cryomicroscopy comes from being able to freeze anything. You can freeze pieces of tissue, you can freeze solutions of molecules, suspensions of viruses, you can freeze ribosomes, you can do virtually anything. And so now electron cryomicroscopy covers a span of about four or five different types of specimen. Each one of these are interesting in themselves and give you an insight into different types of biology.
The simplest one is to freeze a suspension of what we call now single particles. For example, if you take ribosomes, the site of protein synthesis from different types of cells, you can freeze a thin film of ribosomes, you see all ribosomes individually imaged in different orientations. You can pick out the ribosomes and if you have enough of them, 10 or 20 thousand at the moment, you can average all those different views of the ribosome and come up with a 3D structure.
Much as you do tomography, for example, if you have a brain tumor and you go into a hospital, you take x-rays from different angles and you reconstitute get a three-dimensional image of your brain, your eyes, and the tumor. So we do the same thing in structural biology using electronic cryomicroscopy. And you can do it with single particles, without any symmetry, but then you have to take views from all the different angles.
A second specimen are structures in biology with some symmetry. For example, viruses (many of them) have spherical particles that have icosahedral symmetry. That means they have fivefold, threefold and twofold axes that go through the particle. And in each of the icosahedral particles you have 60 copies, 60 different views. So you get one view of an icosahedral particle and essentially you’re looking at the subunits that make up that virus from 60 different directions. So to find this 3D structure of a virus you need 60 times fewer views than you need of a single particle, it’s the same size.
And then you can get many structures in biology that form helical arrangements. The alpha helix is one, actin filaments that are in muscle. You have the thin filaments and the thick filaments in the muscle, and they pull against one another, and your muscles contract. These can be studied very well by electron cryomicroscopy. We see these strands with helices, you take pictures of them, you average them. The way you do the averaging just involve the different geometry, so single particles – you have to find the orientation, a helix – you have a predicted geometrical arrangement of one subunit to the next.
And then the next level of arrangement upwards is a two-dimensional crystal, where you have a single layer, where you have a direction for one axis and another direction to the a-axis and to the b-axis, and this is called electron crystallography. And that was easier initially, because you have in one crystal perhaps 10 thousand molecules in the same orientation.
The final one is when you go away from single particles helices or 2D crystals, you go to general structures. And then people do what’s called electron cryotomography. That means you take a single specimen with no repeating and multiple structures, you take views from all the different angles, say, minus 70 to plus 70 degrees, and then you do a tomogram just like we had without a brain tumor. That gives you the range of different specimens that you can do using electron cryomicroscopy.
Prior to 2010, you could do big structures at high resolution, you could do little structures, but they were tended to be blobs. And electron cryomicroscopists were termed in a derogatory way blobologist, but now we have the resolution revolution, and now all of the structural biologists are piling into electron cryomicroscopy.
So it’s now become a very powerful method that people who have no background whatsoever in an electron cryomicroscopy are joining the field, and there’s a big increase in the number of research departments, universities, research institutes that are investing in electron cryomicroscopy. Now there aren’t enough people to staff all the different facilities that people would like to build. So I think we can say that the electron cryomicroscopy has now become a very powerful method, perhaps, the dominant method in structural biology.

Neuroscientist Onur Güntürkün on mammalian brain, ravens’ ability to plan into future and why birds are so int...

Harvard Associate Prof. Shamil Sunyaev on protein evolution model, human disease mutations, and the help of di...
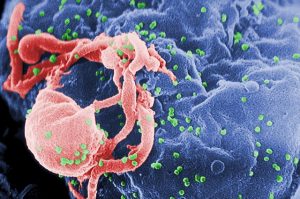
Molecular Biologist Greg Towers on how HIV is transmitted, the possibilities of an HIV vaccine, and how it hel...