The Evolution of Sperm Competition
Harvard Professor Hopi Hoekstra on sexual selection, an optimal clump size, and the unique morphology of the s...
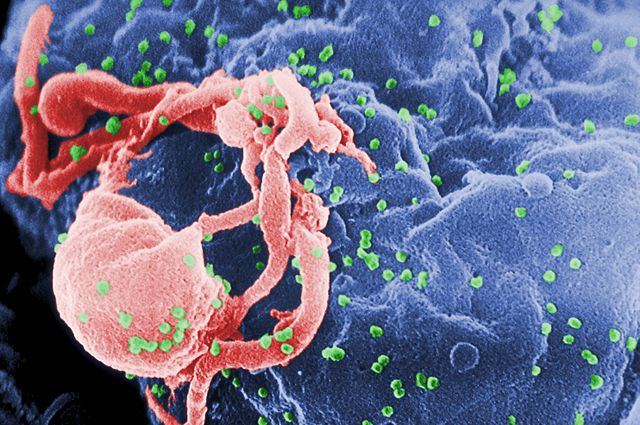
HIV is a human virus and stands for Human Immunodeficiency Virus. It has this name because infection with HIV causes a failure of your immune system called immunodeficiency. HIV is a retrovirus. It is a particular type of retrovirus called a lentivirus. This name derives from the Latin for slow “lentus” because lentiviruses cause a slow disease. It can take many years before an infected person becomes ill with HIV infection. Some people may never become sick, although this is rare. HIV carries its genetic code in the form of RNA inside the viruses. Once the virus gets inside your cells, it turns the RNA into DNA. Our cells can make RNA from DNA, but they can’t make DNA from RNA. That’s why HIV is a retrovirus because it does this backward. HIV is important to study because around 60 million people have been infected and if untreated, almost all would die as a result.
The name “HIV” was first suggested by a committee chaired by Harold Varmus in 1985 and became generally accepted in 1986. The most common form of HIV is called HIV-1, but there is also a rarer form of HIV called HIV-2. The origin of HIV-2 was discovered first in 1992 by the labs of Beatrice Hahn and Paul Sharp. It was discovered later that the common form of HIV-1 came from chimpanzees in 1999, again by Hahn and Sharp. Rarer types of HIV-1 were identified in 2015 to have come from gorillas by Martine Peeters. We know that chimpanzees/gorillas and sooty mangabeys gave HIV to people, rather than people giving the virus to them because the chimpanzees/gorillas and sooty mangabeys have more types of HIV than we do.
There are four types of HIV-1 found in humans, but only 1 type is common. HIV-1(M) has infected around 60 million people. The next most common type HIV-1(O) has only infected about 100,000 people and the last 2 types, HIV-1(N) and HIV-1(P) have only infected a handful of people. HIV-1(M) has evolved to transmit very effectively between people as a sexually transmitted disease, and we don’t understand why this virus can transmit so effectively, whereas the other viruses do not. It suggests that it is very difficult for a virus to adapt to human-to-human transmission, even when it comes from a very closely related species, such as chimps. This helps explain why new viruses infecting many humans are rare.

HIV is found in certain body fluids in an infected individual, for example, blood, semen, and vaginal secretions. Therefore, to catch HIV, one has to come into contact with infected body fluids. Commonly this is through sexual contact or sharing contaminated needles for intravenous drug use. In the early days of HIV, some people became infected through contaminated blood products. Universal HIV testing of blood products has stopped this. When an individual becomes infected, the virus enters specific cells in their immune system called CD4+ T cells. The infected T cells are transformed into virus-producing factories, and a large amount of virus is produced. Many T cells are killed in this process, probably because the body tries to get rid of the virus by killing the infected T cells. However, surviving T cells and other parts of the human immune system can continue to fight the virus. After around ten years, the immune system starts to lose this battle and runs out of T cells. We don’t really understand either why the infected T cells die or why an immune system can fight the virus for more than ten years but then begins to fail.
In many individuals, within the first four weeks after HIV infection, the human body reacts vigorously to its first encounters with the virus. Symptoms of this so-called symptomatic acute HIV infection include fever, rash, swollen glands, and malaise. Typically, these only last a matter of days. However, up to half of infected people do not report any symptoms at all. Over time, untreated HIV infection causes Acquired Immune Deficiency Syndrome (AIDS). As the immune system is exhausted fighting the virus, its ability to counter other infectious diseases is increasingly compromised. AIDS describes a spectrum of conditions that result from this.
There are many organisms, bacteria, fungi, and other viruses, that the human body can normally fight off without disease developing. An HIV-depleted immune system loses its ability to do this. Common infections that define AIDS include oral thrush (a fungus infection of the mouth), herpes zoster (a virus skin infection), and tuberculosis (a mycobacterial infection that may affect any part of the body). An HIV-weakened immune system also loses the ability to fight off certain cancers effectively, including lymphoma. People living with untreated HIV often suffer from weight loss, sweating, and swollen glands that may relate to uncontrolled HIV infection itself. Without a functioning immune system, many of the infections and cancers associated with AIDS do not respond to treatments. HIV is lethal without dedicated therapy.
HIV is treated with drugs called antivirals. The drugs target the viral enzymes. Enzymes are molecular machines that perform tasks required for viral infection. For example, the enzyme reverse transcriptase makes viral DNA. Reverse transcriptase inhibitors like lamivudine or tenofovir prevent the virus from making DNA, thereby preventing cell infection. Another enzyme called integrase stitches the viral DNA into the infected cell DNA, and a new class of drugs called integrase inhibitors, for example, raltegravir, prevent this process. Unfortunately, HIV is very good at making mutations that prevent the drugs from working in a process called drug resistance. These new resistant viruses are called escape mutants, and the drugs do not work against them. To prevent this, we treat HIV infection with several drugs at once, for example, in triple therapy. This means that the virus has to become resistant to all three drugs at once in order to survive. This is much more difficult, and typically the virus does not become resistant to triple therapy. HIV treatment is being improved constantly by targeting different parts of the virus machinery and by modifying the drug cocktails used to get the best anti-viral effect. The drugs can also be taken in 1 pill per day, which reduces the pill burden and makes it easier for people to take their treatment regularly.
Unfortunately, although HIV treatments can prevent the virus from infecting new cells, they cannot completely get rid of all the virus in your body. Therefore, if an infected person stops taking anti-viral drugs, then eventually, the virus will come back and cause disease. This means that you have to take anti-HIV drugs for your whole life. It is difficult to develop anti-HIV drugs because they have to be very effective against the virus but also very well tolerated so they can be taken for very long periods. However, due to an enormous amount of effort from research, patient, and pharmaceutical communities, there are now many drugs available to treat HIV that are very well tolerated by most of the people that take them. The problem now is that these drugs do not cure HIV. A great deal of research is now focused on how we might cure HIV infection. Research is also re-purposing drugs that have hitherto been used as treatments for established HIV infection for use as prevention against acquiring HIV in the first instance. This is called pre-exposure prophylaxis or PrEP. Treatment as prevention (TAsP) is also an important goal. Treating all HIV-infected people will prevent new infections because treatment typically reduces the amount of the virus to levels that cannot be passed on to another person. Effective treatment can, therefore, significantly reduce the number of new cases of HIV infection.
Source: United Nations AIDS Fact Sheet

Cells are very dangerous places for viruses. Over millions of years, we have evolved to protect ourselves from viral infection. This is why we cannot be infected by almost all of the viruses that exist in the world. Only a very small number of viruses are able to infect us, and this is because these particular viruses have evolved to subvert, evade and antagonize all of our defensive systems well enough to infect us. For example, our defensive systems rely on antiviral proteins called restriction factors. TRIM5α is an antiviral protein that coats incoming retroviruses and prevents them from entering the nucleus. It also activates an alarm system to warn nearby cells that the virus is around and then degrades the virus by recruiting the cellular garbage disposal system, called the proteasome.
Another restriction factor, called tetherin, tethers new viruses trying to leave the infected cell, thereby preventing infection of new cells. Instead, the tethered viruses are sucked back inside the infected cell to be degraded. Like TRIM5, tetherin also activates an alarm to warn of impending infection. Activating these alarm systems leads infected cells to make soluble messengers called inflammatory cytokines, for example, interferons, that float off and warn uninfected cells to start making restriction factors to protect themselves from infection. Interferon causes the upregulation of around 500 proteins, the vast majority of which are not understood functionally. When this system works, it can completely protect us from viral infection. In order to infect humans, HIV must bypass these systems.
HIV is invisible to human TRIM5α because its outside surface (capsid) does not stick to human TRIM5α. However, monkey TRIM5α proteins have evolved to be slightly different, and they work effectively against HIV-1 protecting African monkeys from HIV infection. In the case of tetherin, HIV-1 has evolved a gene called Vpu, which targets tetherin to be broken up, leaving the cell vulnerable to HIV infection. Again, monkey tetherin proteins have evolved to be different and are not sensitive to HIV Vpu, making monkeys immune to infection by this virus. Studying the evolving interplay between HIV and mammalian immune systems, referred to as the Red Queen arms race between host and virus, has been enormously helpful in understanding how all viruses work and how we protect ourselves from infection. Of course, all human pathogens have to overcome the same human immune system, and therefore the better we understand this system, the better we can develop treatments and vaccines for all infectious diseases. Importantly, our immune system also protects us against cancer, and a better understanding of immunology promises to help us treat cancer in addition to viral infection.
HIV is a well-studied virus, but there is a great deal that we don’t understand. Current research focuses on many different questions. Significant questions include, why doesn’t current anti-HIV treatment lead to an HIV cure? There are several possible reasons for this. One explanation may be that some virus infects cells in a place where the drugs cannot reach. This may, for example, be in the glandular tissue in the gut. A further explanation for the elusiveness of an HIV cure may be that the virus infects cells that then become inactive. If the cells are inactive or latent, then the virus is not sensitive to the drugs because it isn’t going through the processes that the drugs target. If the cells become active after the drug has been stopped, the virus also becomes active and begins to infect new cells. The cells that are not eradicated by treatment or immunity are referred to as the reservoir. Recent work from Robert Siliciano and collaborators has shown that the reservoir is not static, providing evidence that reservoir cells are constantly being killed and replaced by the immune system. Eventually, anti-HIV treatments may be complemented by drugs that enhance the immune system’s response to HIV. Strategies to activate the virus in the reservoir so that the immune system can kill the infected cells have been called “shock and kill,” and this field is particularly active in the US. This could mean that a cure is eventually possible, although initial results have been disappointing.

Of course, there is also a great deal of research focused on developing an HIV vaccine. HIV is a relatively simple virus; for example, it only has nine genes, whereas the herpes virus has around 200 genes. This simple structure means it has evolved to avoid the immune system very effectively, and this property likely explains why we have failed to develop a vaccine that triggers a protective immune response. Pioneering work by Louis Picker’s lab has developed a vaccine candidate based on putting lentiviral genes into the DNA virus cytomegalovirus (CMV). In monkey models, treating animals already infected with the simian version of HIV (SIV) with the CMV/SIV chimeric virus leads to about half of the animals apparently being cured of SIV infection. It’s not clear why this is so effective, but these studies will help us understand the kind of immune reaction we need to induce for a cure/vaccine and may lead to a similar human-based chimeric virus that can be used for treatment or vaccination.
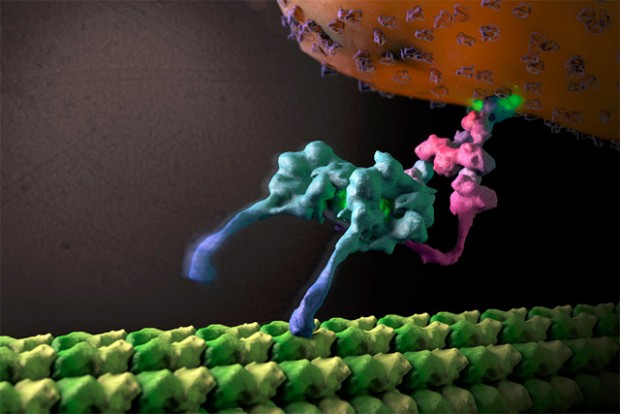
HIV acts as a model for many viral infections, and studying HIV helps us understand how all viruses work. This is particularly true when studying the interaction between viruses and the immune system. For example, recent work from Leo James’ lab has shown how the HIV-1 capsid, which acts as a cloak to protect the viral RNA and DNA from the cells’ defenses, acts as an electrostatic channel that sucks in the nucleotide fuel for viral DNA synthesis. This is important because it provides a paradigm for how viruses hide their nucleic acid while synthesizing new genomes. Disrupting this process using drugs can be used as a new way to treat viral infection because disturbing viral cloaking makes the virus visible to the immune system leading to a very powerful antiviral effect that can completely suppress viral replication.
The virus has a great deal to tell us about human biology. Developing and employing tools to follow its journey through the body and through our cells reveals new knowledge about the human immune system and human cell biology that will fundamentally improve our understanding of infection, cancer, and autoimmune disease. All this new knowledge will underpin the development of treatments for these diseases.
In the clinical setting, there remains great public health work ahead. Late diagnosis of HIV infection and late initiation of anti-retroviral treatment continue to cause many thousands of deaths worldwide. Health education, effective diagnosis, and improved worldwide health infrastructure remain key global priorities. One evolving clinical narrative concerns non-communicable diseases. There is a strong association between HIV and increased rates of heart disease, stroke, and kidney disease, which among other non-infectious conditions, contribute to increased morbidity and mortality in HIV-infected individuals despite effective anti-viral treatment. There is essential descriptive work to understand the burden and natural history of these diseases in the HIV-infected population. This will lead to basic science research that may translate to potential interventions. This new knowledge may help us understand and treat these conditions in patients without HIV infection, which would have an extraordinary global impact on human health and disease.
Gao, Feng et al. (1999) “Origin Of HIV-1 In The Chimpanzee Pan Troglodytes Troglodytes.”. Nature 397.6718 : 436-441. DOI: 10.1038/17130. PMID: 9989410
D’arc, Mirela et al. (2015) “Origin Of The HIV-1 Group O Epidemic In Western Lowland Gorillas”. Proceedings of the National Academy of Sciences 112.11 : E1343-E1352. DOI: 10.1073/pnas.1502022112. PMID: 25733890.
Deeks, Steven G et al. (2016)”International AIDS Society Global Scientific Strategy: Towards An HIV Cure 2016″. Nature Medicine 22.8 : 839-850. DOI: 10.1038/nm.4108. PMID: 27400264
Jacques, David A. et al. (2016) “HIV-1 Uses Dynamic Capsid Pores To Import Nucleotides And Fuel Encapsidated DNA Synthesis”. Nature 536.7616 : 349-353. PMID: 27509857
Stremlau, Matthew et al. (2004) “The Cytoplasmic Body Component Trim5α Restricts HIV-1 Infection In Old World Monkeys”. Nature 427.6977 : 848-853. DOI: 10.1038/nature02343. PMID: 14985764
“Fact Sheet 2015 | UNAIDS”. (2016) UN AIDS. http://www.unaids.org/en/resources/fact-sheet
Edited by John Mark Shorack

Harvard Professor Hopi Hoekstra on sexual selection, an optimal clump size, and the unique morphology of the s...

Geneticist Steve Jones on why DNA is a language, how biology was invented as a science and why Charles Darwin ...
New study describes genealogy and spreading of one of the most damaging species of pest ants