Fire ants conquer the world on the first global tr...
New study describes genealogy and spreading of one of the most damaging species of pest ants
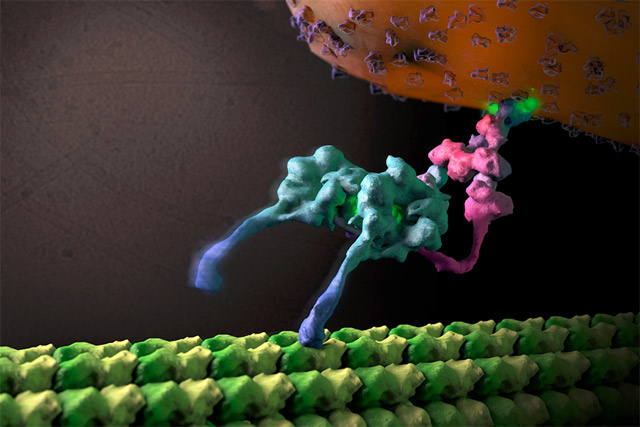
In March 2015 Nature Structural & Computational Biology published an article “Structural organization of the dynein–dynactin complex bound to microtubules” about the newest research on the way motor proteins transport cargo along the molecular track. We have asked one of the authors of this research, Dr. Saikat Chowdhury from the Scripps Research institute, to comment on this work.
The growth and socio-economic development of nations is dependent on their ability to transport cargoes by various types of freight carriers. Similarly, our cells have established transport systems that carry a wide range of molecular cargoes on great distances along specific tracks. This movement of cargo is crucial for cellular growth, survival, and viability, and is primarily carried out by two types of motor proteins that move within cells in opposing directions – kinesin and dynein. While much is known about kinesin, the way that the dynein transporter complex travels within the cell remains very much a mystery. Using transmission electron microscopy and advanced image processing algorithms, we were able to describe the complete structural organization of native cytoplasmic dynein, showing how its many components are assembled to produce this powerful machine.
Furthermore, we were able to trap the dynein motor as it was moving along its molecular track (thin, hollow cylinders called microtubules) to get a snapshot of its organization during cargo transport. This is akin to taking photographs of horses mid-race during the Kentucky Derby, revealing how various structural components are positioned and arranged to achieve efficient directional motion. In order to become a unidirectional fast-moving motor, a large cofactor named dynactin must bind to the dynein to form the dynein-dynactin supercomplex.
This study provides important new insight into the structural basis of the dynein-dynactin interaction as this super-complex walks along the microtubule surface. When dynactin is attached to dynein, the dynein motor domains are positioned in close proximity to one another at the front of the complex, attaching to the microtubule in a similar forward-facing orientation. This organization likely promotes consistent, unidirectional movement of the motor domains, while a variety of other regions within both dynein and dynactin are located further away from the microtubule surface and made accessible for attachment to molecular cargo.
These findings provide a structural framework for understanding dynein-dynactin-mediated cargo transport, and for interpreting decades of biophysical and biochemical work aimed at deciphering the mechanics of dynein motor. However, this is a first fundamental step in understanding this system, and many questions remain. We plan to build on the findings of this study to explore how this machine carries large enormous vesicles and organelles microns in distance along the cellular interior. Do many copies of this complex work together to haul cargo? How is their movement influenced by other regulatory cofactors? How are these machines loaded onto the microtubules? And importantly, we are very interested in how these motors behave in the presence of the protein aggregates found in the neurons of Alzheimer’s and Parkinson’s patients. Through further examination of this complex in presence of a variety of cellular cargoes and other adapter proteins, and pursuing higher resolution 3D studies, we hope to establish a holistic understanding of the underlying mechanisms that drive this fascinating transporter complex.
New study describes genealogy and spreading of one of the most damaging species of pest ants

Biogerontologist David Gems on the senescence, medical approach to aging and how can you research the fundamen...

Biophysicist Georg Büldt on using fluorescent protein markers, hormonal communication during pregnancy, and in...