Treatment of Aging
Biogerontologist David Gems on the innate plasticity in aging, senolytic drugs and whether we should expect dr...
The video is a part of the project British Scientists produced in collaboration between Serious Science and the British Council.
HIV is the first human retrovirus which is a particular class of viruses that are known to exist in other species, and HIV is the first example of one of those viruses in humans. HIV was first identified in the early 80s when there was a series of cases of gay men turning up in hospitals in emergency rooms with a new illness that appeared to be related somehow to immunodeficiency. Their immune systems were failing and it really wasn’t clear why that was and it took some time to work out that this was a new viral infection that hadn’t been seen in humans before. It was first described in the early 80s in San Francisco and then it was realised that it was more widespread than that. The people who identified it worked in the Institute Pasteur. So Françoise Barré-Sinoussi and Luc Montagnier had a lab working on other retroviruses and they discovered that, they looked down a microscope essentially, an electron microscope, and they could see that there was a retrovirus in the cells from the patients who had this disease.
They won a Nobel Prize for that work, a lot of other people contributed in the US, for example, Robert Gallo. And then a test was developed and we could test who had the virus, it turned out that quite a large number of people had the virus and at that early point there was no treatment. So it turned out that it is spread through sexual transmission, it’s a sexually transmitted disease, it can also be spread through blood products and in the early days before screening people who were taking blood products as a medicine, for example, people with hemophilia, were infected with the virus. You could be infected through blood contact, for example. So intravenous drug users also are very susceptible to catching it because there a tendency to share needles, and so if one person was infected that would infect everybody who shared the needle. So in those early days, it was very frightening because it was a new virus and people were dying and nobody knew what to do about it. But luckily we knew quite a lot about retroviruses because retroviruses have been described in other species particularly mice, so it was reasonably quickly for the development of a new type of medicine to come up with ways to treat HIV and that treatment advanced to nowadays.
In 2016 you can really treat the virus with a cocktail of antiviral drugs that will stop it infecting new cells in your body theoretically. And that means that the virus becomes undetectable in the vast majority of cases and we think that you can probably live a normal lifespan if you take your antiretrovirals. That’s fine if you can afford the antiretrovirals so in West that is great, in a resource-poor settings like sub-Saharan Africa that’s more problematic where there is less money available to buy antiretrovirals which can be quite expensive. But they are available and there are large programs to roll out antiretroviral treatment with a goal of making sure that everybody who has the disease has access to antiretrovirals.
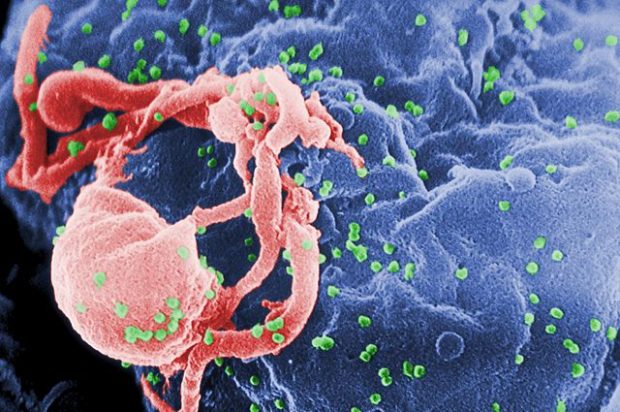
HIV is the human retrovirus, it stands for Human Immunodeficiency Virus, there are several versions of it and the most common of it is called HIV-1. So AIDS is acquired immunodeficiency syndrome and that is a disease that’s caused by being infected with HIV. And as far as we can tell, we believe that pretty much everybody who becomes infected with the virus will eventually get a disease so it’s extremely rare for somebody to never get a disease and it’s very difficult to say whether somebody would never get it – they might die for some other reason, but the vast majority of people suffer from disease but not until about 8 to 10 years after they’ve become infected. So there is a long period where they don’t really suffer significant symptoms, although there are symptoms.
When you first become infected with HIV, you get infection syndrome very similar to other viral infections, for example, influenza. So the virus replicates in your body to very high titers, very large amounts of virus in your body, and it makes you feel quite ill. You might have a rash and a temperature and you might spend a few days in bed and you feel like you’ve had some kind of viral infection. And then it goes away, and your immune system suppresses viral replication. And it can suppress it to very low levels so that you can’t actually detect the virus in your body after that first couple of weeks of infection.
So what happens after that it’s not entirely clear but what’s certainly true is that your immune system is fighting an ongoing battle with the virus and you don’t feel particular symptoms during that period so there is sense that you’ve got better, you’ve recovered from the virus but that isn’t the case in the case of HIV. It’s is still in you body replicating. And that means that eventually for reasons that we don’t fully understand your immune systems runs out of energy or runs out of steam and it starts to fail and the virus gets the upper hand and destroys your immune system and this long battle between the virus and immune system leads to a complete failure of your immune system and that’s why you get the disease and the disease of HIV is largely the disease of opportunistic infection.
So the illnesses that you would not normally suffer, for example, fungal infections, we’re constantly exposed to fungi that would like to eat us up but they don’t because our immune system protects us from it. But without an immune system, those fungal infections start to cause very bad infections in your mouth and in your lungs and your airways. And when AIDS was first described those kinds of diseases haven’t been seen in humans before because we’re very good at fighting them using our immune system, so it was very difficult to treat them. Nowadays much more treatments have been developed for those diseases. But there is a long period between 8-10 years median when your body is fighting the virus and keeping it in check.
There is really two possibilities for that, I think and it’s currently a lot of research trying to work out which of these two possibilities is true, possibly both. Possibility number one is that the virus is in your body somewhere in the organs perhaps in the gut in the lymphoid tissue and it’s replicating there just at low levels. Another possibility is that it really does stop replicating and the drugs get rid of all of the cells that are making virus and the only cells that are left in your body are cells that got the virus in them but that aren’t actively making virus and so can’t get rid of those guys until they start making virus. So if you stop taking the medicine sooner or later those cells will start making a virus and the whole process will start again. So we don’t really understand whether we need to make the drugs better to kill the virus, the last little bit of virus or whether we need a new strategy to wake up the cells that are quiet and not making the virus, so we can kill them as well. And that essentially constitutes the cure research agenda that is currently trying to work out the best way to actually cure people.
There are two broad areas of HIV research. So particularly in the US, I think now there is a very strong effort to focus everybody’s research onto the cure. There is a sense that we shouldn’t be fooling around, we should just focus on curing the patients that are infected and that is really the only way to eradicate the disease. There is also, of course, a big effort to make a vaccine, but we just don’t understand how to make a vaccine and we just don’t understand enough about vaccines as to understand why can’t we make an HIV vaccine. The things that have been tried are the classical ways to make a vaccine and they simply don’t work for HIV.
There are lots of possible reasons for that but we don’t really understand why. And I don’t think that anyone really believes that there is going to be a vaccine anytime soon, hence I think the shift towards the notion of perhaps we can start to think of new ways to cure people, perhaps with new therapeutic strategies. So that’s one big area focused on research.
The second broad area of HIV research, I think, is using HIV as a tool. HIV is a fairly small virus, it only has nine genes, so it makes nine proteins. Some of those proteins are quite complex and they’re broken up into other proteins, but it’s a simple virus. A Herpes virus, for example, would have more than 200 genes, whereas HIV only has nine. So that makes it a very tractable genetic tool.
We can use the virus to study cell biology and that’s been a very powerful way to understand what’s going on inside the cells of our body, how do they work, how do they divide, how does the stuff move around, how are they organised and HIV is a fantastic tool for doing that. For example, we’ve learned a great deal about RNA export from the nucleus, how is the RNA export from the nucleus regulated, how is splicing regulated. HIV has to manipulate these processes and studying how it does that told us a great deal about that. We’ve understood a great deal about transcriptional control, the virus carries its own transcriptional activator, a protein called Tat and how that works is quite different from other transcriptional activators and studying it told us a great deal about how transcription works. So it’s a fantastic tool to use for various scientific questions, so we in my own laboratory use HIV really as a tool to study the innate immunity.
Studying viral infections in general is very important because there is a possibility that as we understand more about how viruses replicate and particularly about how cells typically protect themselves from infection and how viruses get over those protective strategies, we can start to understand that there are certain things that many different viruses have to do in order to infect human cells. And if we start to drug those processes, there is every chance that you can start to develop antivirals with much broader specificity. So you might be able to develop drugs that hit multiple viruses with a single drug. I think that is something that’s only really now becoming a realistic prospect, but it’s something that we feel very enthusiastic about.

Biogerontologist David Gems on the innate plasticity in aging, senolytic drugs and whether we should expect dr...

Neuroscientist Karl Friston on the development of the free energy principle, what remains unknown about the br...

Neuroscientist John Krystal on alcohol's action on the brain, social and habitual drinking, and why some peopl...