Photodynamic Therapy of Cancer
Chemist David Philips on the phenomenon of phototoxicity, characteristics of the dyestuff, and limitations of ...
Today, fossil fuels—oil, gas, and coal—account for over 80% of the global energy balance [1]. The remaining 20% comes from alternative energy sources: nuclear power plants, solar, hydro, wind energy, and biofuels.
Fossil fuel reserves are finite, and their combustion releases carbon dioxide, contributing to the greenhouse effect and playing a significant role in climate change. This reality drives humanity to expand the use of existing alternative energy sources while seeking new methods for storing, transporting, and converting energy. One such solution is hydrogen energy.

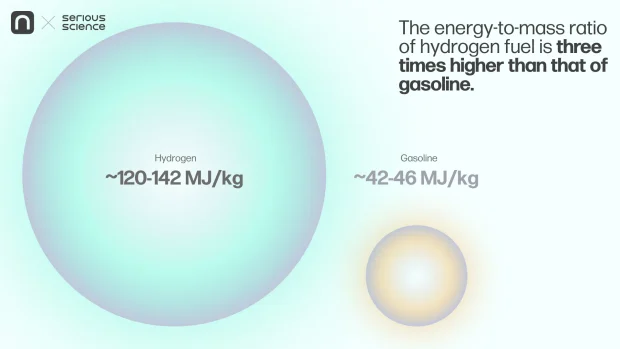
Hydrogen is the most abundant element in nature. It is a gas that releases a significant amount of heat when burned. The energy-to-mass ratio of hydrogen fuel is three times higher than that of gasoline. Hydrogen energy conversion methods are diverse, simple, efficient, and environmentally friendly compared to traditional energy systems. For instance, hydrogen oxidation generates electricity and water—a valuable and clean resource. This makes hydrogen an integral part of a new energy paradigm.
Hydrogen has numerous applications. It can serve as fuel for vehicles and public transport powered by highly efficient hydrogen fuel cells, which produce zero carbon dioxide emissions. Hydrogen can also replace coal and other fossil fuels in heating systems. Excess electricity generated by solar and wind installations during low-demand periods—such as at night, when most people are asleep—can be stored using hydrogen. This is achieved by directing the “surplus” energy toward water electrolysis, splitting H₂O into hydrogen and oxygen. The resulting hydrogen can be used during windless or overcast conditions when wind turbines and solar panels produce less energy.
Despite its potential, hydrogen energy comes with its own set of challenges. The first issue is that hydrogen is rarely found in its pure form on Earth—it must be extracted from various chemical compounds, a process that requires significant energy. One of the “cleanest” methods to produce hydrogen is through electrolysis, which demands substantial electricity. Another method involves breaking down methane at high temperatures.
Additionally, hydrogen is an extremely light and poorly liquefied gas, making it difficult to transport. Currently, hydrogen must be transported either in chemically bound forms, as a compressed gas in high-pressure cylinders, or in liquefied form. Special safety measures are essential during transport, as hydrogen forms explosive mixtures when combined with air.
These factors result in considerable financial and energy costs. Many companies initially inspired by the potential of hydrogen energy have slowed down the implementation of these projects for economic reasons. Nonetheless, there is a consensus within the scientific community that these challenges are solvable and that the future belongs to hydrogen energy. Researchers continue to explore ways to transition from fossil fuels to hydrogen-based systems.

Burning hydrogen is not an entirely clean process. The air used during hydrogen combustion always contains nitrogen. At high temperatures, nitrogen and oxygen molecules react to form toxic nitrogen oxides, which are also present in vehicle exhaust gases.
Fuel cells address this issue by directly converting the chemical energy of hydrogen fuel into electricity, bypassing the heating stage. The absence of emissions, high efficiency, and quiet operation make fuel cells a cornerstone of hydrogen energy.
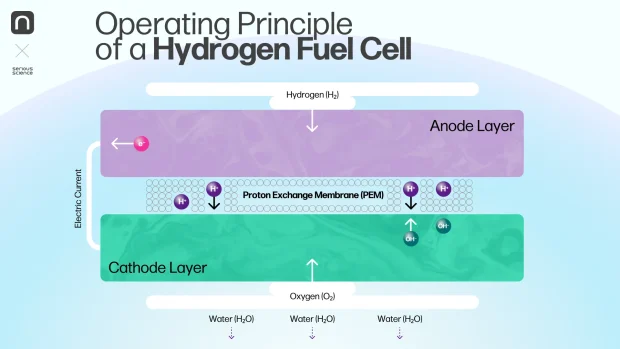
A fuel cell consists of membrane-electrode assemblies (MEAs) [2]. Each assembly includes a polymer membrane flanked on both sides by porous electrode layers—a cathode and an anode. These layers contain a catalyst, a substance that accelerates chemical reactions. While the membrane is nearly impermeable to gases, it allows the passage of tiny particles such as protons or hydroxide ions.
The operation of a fuel cell is relatively straightforward: a reductant (hydrogen) and an oxidant (oxygen) are supplied to the electrodes. At the anode, where hydrogen is introduced, an oxidation reaction occurs. Simultaneously, at the cathode, oxygen undergoes a reduction reaction. During oxidation, hydrogen molecules split into protons and electrons, which take different paths to the cathode. Electrons travel through an external circuit, generating an electric current, while protons migrate through the membrane to the cathode, where they combine with oxygen. This reaction produces heat and water expelled from the fuel cell.
The durability (or stability) of fuel cells depends on the properties of the catalytic layer—from the design and composition to the structure of individual components—as well as operating conditions. This is a critical parameter: if hydrogen vehicles required fuel cell replacement every two years, they would be impractical. To address this, fuel cell developers sometimes prioritize stability over activity. Modern hydrogen fuel cells are typically designed to last around five years.
Hydrogen fuel cells can be utilized in land, water, and air transport, as well as in unmanned aerial vehicles and space exploration. Today, nearly all major automotive manufacturers—Honda, Hyundai, Audi, BMW, Ford, Nissan, and Daimler—are developing and testing vehicles powered by hydrogen fuel cell systems. Russia’s “KAMAZ” has also introduced its hydrogen-powered truck with a payload capacity of over 20 tons, capable of travelling up to 500 kilometers on a single refuelling.
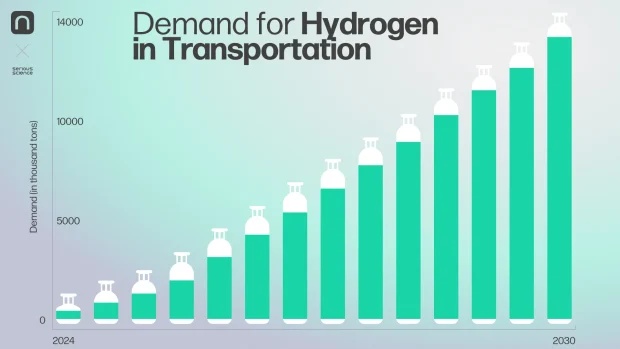
Suppose the targets outlined in the hydrogen transport strategies of the United States, China, Japan, the European Union, and other nations are met. In that case, hydrogen consumption in the transportation sector will increase from 140,000 tons annually to 14 million tons annually by 2030.
One of the key components of a fuel cell is the catalyst. Without this ultra-thin layer, energy conversion would be tens of thousands of times slower, making it impossible to achieve a dense flow of electrons.
Platinum is used as the catalyst in hydrogen fuel cells. Due to the unique properties of its crystal lattice, chemical reactions on platinum occur many times faster than on other metals. However, platinum is an extremely expensive material, making it essential to use in minimal quantities. For example, the hydrogen-powered Toyota Mirai uses only 10–15 grams of platinum [3].
In fuel cells, platinum is applied as nanoparticles onto the surface of a carbon carrier, which conducts electricity effectively. The carbon prevents the platinum particles from clumping together, enabling extensive electrode coverage with minimal material cost. One gram of platinum nanoparticles deposited on carbon can create a catalytic layer with a surface area of up to 120 square meters. This achievement was made by a team of scientists from Southern Federal University (SFU), who founded the company “Prometey RD” to produce hydrogen fuel catalysts [4].
Platinum nanoparticles can take various shapes, as catalytic activity also depends on the crystal’s form. According to some studies, specific combinations of crystal shapes and sizes can increase activity by up to five times, significantly reducing platinum usage. This is crucial given the Earth’s limited platinum reserves—its average concentration in the Earth’s crust is approximately 5×10⁻⁷% by mass [5]. Platinum supplies may prove insufficient if humanity adopts fuel cells and electrolyzers on a large scale.
Researchers worldwide are working to develop entirely platinum-free catalysts to reduce the cost of fuel cells. Such catalysts could enable the use of a different membrane type—anion exchange membranes that transport OH⁻ ions instead of protons. However, these technologies currently lag behind proton exchange membrane fuel cells with platinum catalysts in terms of performance and reliability. As a result, the simplest approach is to reduce the platinum content in the catalytic layer by mixing it with another metal.
In a recent study, scientists from Southern Federal University (SFedU) explored the use of palladium for this purpose [6]. Preliminary research has shown that palladium can enhance the catalytic activity of platinum. While nickel and cobalt can improve platinum catalyst properties, these metals are thermodynamically unstable and gradually “leach” from the catalytic layer. In contrast, palladium is a noble and thermodynamically stable metal that can remain within nanoparticles for extended periods. Experimental platinum-palladium catalysts have shown a lifespan comparable to commercial platinum catalysts.
Palladium’s natural reserves exceed those of platinum, making its use a viable option for increasing the production of catalysts without compromising—and potentially improving—their quality. To date, SFU researchers have studied two platinum-palladium systems. The first is a solid solution where platinum and palladium atoms are mixed within the crystal lattice, albeit not always evenly. The second, more promising system is a “core-shell” structure, where the core consists primarily of palladium atoms surrounded by a platinum shell.
In this structure, platinum resides on the surface of palladium, subtly altering its properties and making it a more effective catalyst. The core and shell size can be adjusted to produce catalysts with unique characteristics. However, microscopic defects can form in the platinum shell, accelerating its degradation. Further research is needed to understand and address these issues fully.
A key challenge remains: how to produce billions of nanoparticles with identical shape, structure, and size. Their synthesis methods must be industrial, scalable, cost-effective, and environmentally friendly. As a result, the microstructure of catalysts requires constant refinement to strike the right balance between performance, cost, and sustainability.
Despite these challenges, hydrogen energy development programs are gradually being implemented in developed countries. Ready-made solutions are already being offered to African nations, enabling them to establish environmentally friendly and efficient systems without relying on outdated technologies.
According to the International Energy Agency, if all proposed projects are successfully implemented, hydrogen use could reduce global natural gas consumption by 14 billion cubic meters per year, coal by 20 million tons per year, and oil by 360,000 barrels per day by 2030 [7]. This reduction would be equivalent to all known fossil fuel reserves in Colombia.
Belgian company Umicore, a producer of platinum-containing electrocatalysts, estimates global demand for these catalysts will reach 24 tons by 2025 and 90 tons by 2030 [8]. Meanwhile, other industries are also advancing, and the list of advantages offered by hydrogen technologies over alternatives such as lithium-ion batteries is being regularly reassessed.
1. bp Statistical Review of World Energy 2022 (71st edition)
2. Vodorodnaya lihoradka [Hydrogen fever]. An analytical review. NRA, February 2022
3. Ne zoloto, a platina: kak uchenye iz YuFU razvivayut «zelenye» tekhnologii [Not gold, but platinum: how the scientists of the SFedU develop “green” technologies]. RBK, October 10, 2024
4. Paperzh, K.O., Alekseenko, A.A., Volochaev, V.A., Pankov, I.V., Safronenko, O.A., & Guterman, V.E. (2021). Stability and activity of platinum nanoparticles in the oxygen electroreduction reaction: is size or uniformity of primary importance? Beilstein Journal of Nanotechnology, 12, 593–606
5. Reith, F., Campbell, S., Ball, A., Pring, A., & Southam, G. (2014). Platinum in Earth surface environments. Earth-Science Reviews, 131, 1–21
6. Guterman, V., Alekseenko, A., Belenov, S., Menshikov, V., Moguchikh, E., Novomlinskaya, I., Paperzh, K., & Pankov, I. (2024). Exploring the potential of bimetallic PTPD/C cathode catalysts to enhance the performance of PEM fuel cells. Nanomaterials, 14(20), 1672.
7. World Energy Outlook 2023 (2023, October 1). IEA
8. Sap, B. (2022). Capturing the emerging growth in fuel cells. UMICore Capital Markets Day 2022

Chemist David Philips on the phenomenon of phototoxicity, characteristics of the dyestuff, and limitations of ...

Biochemist Ian Wilson on toxicity of paracetamol, clinical trials, and safe drugs
What can be used to produce fuel?