Retroviruses in Gene Therapy
Molecular biologist Greg Towers on the great scientific challenges, the dangers of gene therapy, and the way in which it can treat cancer
videos | August 16, 2017
The video is a part of the project British Scientists produced in collaboration between Serious Science and the British Council.
The retroviruses are a fantastic tool to do gene therapy. Gene therapy is a way to treat genetic disease. This is the case where somebody has a mutation in the gene that leads to a protein in the body functioning poorly. If that’s an important protein, that can lead to illness. You can treat that in various ways, but a really good way to treat that disease is to fix the gene defect in the cells that need that protein.
Another use of gene therapy which is becoming increasingly effective is cancer. So cancer cells are doing different stuff to your normal body, the cells in your body, they’re dividing very rapidly. They’re sucking up a lot of metabolites, the tumor might be growing blood vessels and stuff like that. So you can put in gene therapy to change the immunology of the tumor, so that your immune system can see the tumor again, where the tumor has shut down its ability to be seen by the immune system. You can fix that with gene therapy.
Gene therapy essentially involves the ability to infect your target cells with a virus, and that virus must contain the gene that you want to put in there to either fix them or to make the tumor visible to the immune system, for example. You can use retroviruses for gene therapy, because you can firstly make viral particles with the genome inside that only contain your favorite gene, and you can then infect your target cells. Those infected cells will only be modified by the insertion of your target gene into their chromatin. That’s great. And retroviruses are particularly good at that, because they insert it into the chromatin. So once the gene is there, it’s stable.
With all gene therapy the rate-limiting step is simply the ability to put the gene into the right cells. The gene therapies that work best are often the ones where you can either inject the virus directly into the site (so, for example, into a tumor), or the case where you can take the cells out of somebody’s body (for example, their blood cells or their bone marrow cells), modify them by infecting them with the viral vector, and then put them back in their body. In that way there’s a relatively straightforward way to modify people’s cells with a gene therapy vector that’s derived from a retrovirus.
In the early days, gene therapy was carried out with the mouse gamma retroviruses. That was taking a mouse virus, taking out the mouse virus genes, putting in your gene and then using that to deliver the gene. That’s worked very well. And there are still gene therapy trials going on using the mouse virus. A problem with the mouse retrovirus is: it will only infect cells that are actively dividing, and there are many cells that you want to infect that aren’t particularly dividing (stem cells don’t divide very well and the variety of other blood cells that don’t really get infected with the gamma-retroviruses).
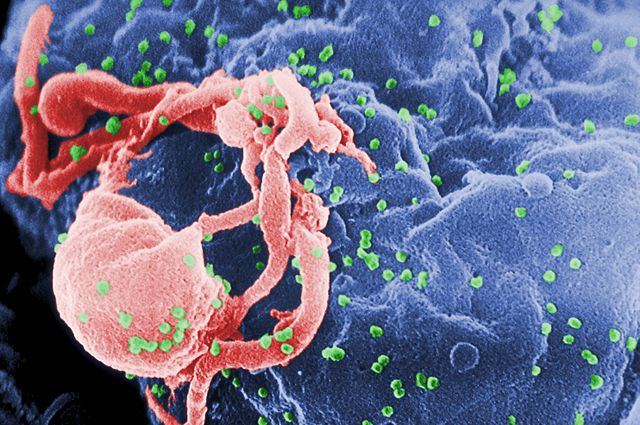
More recent therapies are using HIV-1-derived viruses. You can imagine that that can cause a problem, because you don’t want to infect somebody with a virus that’s going to cause them any additional disease. There are a lot of safety concerns with retroviral vectors, and people have been very careful to disable the virus in multiple ways, so that you can make it safe. Of course, disabling the virus makes it less infectious. So, there’s a constant trade-off between how safe the virus is and how effective it is as a gene therapy vector to deliver a therapeutic gene.The history of gene therapy has really gone from developing the vectors to be more sophisticated, safer and more infectious. The other problem is engraftment. So, you have to be able to put the modified cells back in the person’s body and not have those cells immediately die. Testing various ways to do the infection, how you look after the cells very carefully after you’ve taken them out the person’s body, how long you keep them out of the body, what media you grow them in and stuff like that. It’s a massive industry with people trying to work out the best way to take the cells out and put them back in without damaging them in any way.
One of those exciting gene therapies that are going on at the moment is what is called CAR or chimeric antigen receptor. That’s based on the idea that when you get a cancer – I think, everyone agrees now that cancer is something that is really fought by your immune system. During your life cancers are forming on an individual cell basis, but they’re killed. So, cells do strange things, and your immune system recognizes something funny is going on and kills them. In order for a tumor to survive in your body and grow, it has to change in such a way that your immune system can’t see it. That is bad news. If your immune system can’t see it, the tumor can grow.
The idea of CAR therapy is that you can take the cells out of somebody’s body, their T-cells, which are the cells that might be killing tumor cells, and you can modify their specificity, so that they can see the tumor again. They do that by making what that is referred to as chimeric antigen receptor. Essentially, the T-cell receptor, which is on the surface of the cell, swimming around looking for things to kill. You change that molecule for an antibody that specifically recognizes the cancer, and you fuse that antibody to the other half of the T-cell receptor, the part that’s inside the cell. When the antibody on the surface of the T-cell binds the CAR, the chimeric antigen receptor, then it activates the T-cell to kill the tumor. And that’s a fantastically effective therapy when it works. There are people who’ve been treated in 2015-2016, children with leukemias that would not be treatable in any other way, and yet the CAR therapy can give them effectively a cure. So, that’s a really exciting area of gene therapy that’s being developed at the moment. It’s really starting to work.
Because gene therapy is a way to cure a genetic lesion, sometimes it’s the only way to treat a particular disease. There might not be any other strategy to do that. So, one of the real values of gene therapy, I think, is it brings a whole new series of treatment options. Unfortunately, as it develops it’s very expensive and it’s very bespoke. So, you often find that you can only treat a small number of patients per year, and you might have to have that person at the hospital, might have to take cells out of their body, you might have to make a treatment that is really specific to their disease. So, at the moment gene therapy is not something that can be mainstream, that can be used to treat thousands of patients in the way that surgery or other treatments can. I think that’s one of its great disadvantages. But as we develop gene therapy, there is every chance that it can become mainstream therapy.
I think, gene therapy really is the future for many things, not just genetic diseases. For example, there are ways to try and treat HIV infection with gene therapy. And the idea would be that you might try, and you might take cells from someone’s immune system out of their body, and then make them insensitive to HIV infection by, for example, stopping them making the HIV receptor CCR5, or by expressing an antiviral gene that is a part of your innate immune system. A modified innate immune factor – put that inside the person’s T-cells, put those T-cells back in their body, and then those t-cells, those modified cells won’t be infected by the virus in their body. So, those cells can fight the viral infected cells. It’s possible theoretically that that could be a one treatment cure, but we’re a long way from that. It’s about improving our ability to deliver the genes, it’s about selecting the right genes to treat each particular disorder, and it’s about getting better at the actual delivery of these genes to improve the efficiency of delivery and the efficiency of engraftment, so the maximum number of cells is returned to the patient effectively.
There are some risks with gene therapy, and there are cases where people who’ve been infected with gamma-retroviruses encoding a therapeutic gene have ended up getting cancer. It’s not entirely clear why that is. It’s a combination, I think, between the fact that the gene, the therapeutic gene, had certain properties and the gamma-retrovirus had certain properties, and when those properties are put together that significantly increases your risk of getting cancer.
Those studies that discovered cancer and children that have been treated for immune deficiency syndromes, those studies were very worrying, because people thought maybe this will kill gene therapy, maybe we’ll be in a situation now where the risks can still be too great. So, it’s very important to do gene therapy in a different way, in a way that will not cause cancer. I think that that is achieved by using a slightly different therapeutic gene and by using a different vector. The shift to using HIV-based vectors is driven in part by the fact that they are less likely to cause cancer, because HIV typically does not cause cancer.

One risk in the early days was that if a person who’s been treated with gene therapy using an HIV vector, what if they get infected with HIV through the normal sexual transmission. That may lead to a situation where the virus that’s in their body contains a mixture of genomes, the genomes of the virus that infected them, plus the genome of the gene therapy genome. Then they could infect another person and that modified genome could transmit to them. So, people have worked out a way to stop that happening. There are bits of the virus that you can cut out, and the virus will still work, but a new virus showing up cannot package that into viral particles. They’re called self-inactivating mutants, and they’re very safe.
So, one of the problems of doing gene therapy with retroviruses, particularly with HIV, is that we have to transfect cells every time we do it to make the vector. That means, we have to get a cell line in the lab, and we have to put it into flasks and we have to introduce the viral DNA into the flasks fresh every time. That makes it very expensive, and each time you do it you have to check that that particular prep is safe, and you have to do lots of regulatory tests on it, which uses up some of the prep and you have to remake it every time you want to treat the patient.
What we would like to do is to develop a cell line that contains the gene therapy vector and constantly produces it. Then the virus that’s coming out of those cells could be used as a medicine, and you can grow those cells for a period of time and keep making fresh virus. That requires to develop a cell line that consistently produces large amounts of retroviral vector. That is one of the Holy Grails of the gene therapy field to develop this kind of reagents that will constantly provide a consistent and a safe source of the retroviral vectors put into people’s bodies. I think, that’s one of the great challenges.
Another great challenge is the developing each gene therapy case. Each disease will require a new gene, so you have to figure out which gene you want to use, what strategy you want to use in order to treat a patient. You need to be able to infect the right cell. There’s a great deal of work goes into developing new ways to use established gene therapy techniques to treat new diseases. I think, as I said earlier, one of the most exciting features of that work is the use of gene therapy vectors to treat cancer.